Potassium azide
| |||
 | |||
| Names | |||
|---|---|---|---|
| IUPAC name
Potassium azide | |||
| Identifiers | |||
| 20762-60-1 | |||
| 3D model (Jmol) | Interactive image | ||
| ECHA InfoCard | 100.039.997 | ||
| PubChem | 10290740 | ||
| |||
| |||
| Properties | |||
| KN 3 | |||
| Molar mass | 81.1184 g/mol | ||
| Appearance | Colorless crystals[1] | ||
| Density | 2.038 g/cm3 [1] | ||
| Melting point | 350 °C (662 °F; 623 K) (in vacuum)[1] | ||
| Boiling point | decomposes | ||
| 41.4 g/100 mL (0 °C) 50.8 g/100 mL (20 °C) 105.7 g/100 mL (100 °C) | |||
| Solubility | soluble in ethanol insoluble in ether | ||
| Thermochemistry | |||
| Std enthalpy of formation (ΔfH |
-1.7 kJ/mol | ||
| Hazards | |||
| Main hazards | Very Toxic, explosive if strongly heated | ||
| NFPA 704 | |||
| Lethal dose or concentration (LD, LC): | |||
| LD50 (median dose) |
27 mg/kg (oral, rat)[2] | ||
| Related compounds | |||
| Other cations |
Sodium azide, copper(II) azide, lead(II) azide, silver azide | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Potassium azide is the inorganic compound having the formula KN
3. It is a white, water-soluble salt. It is used as a reagent in the laboratory.
It has been found to act as a nitrification inhibitor in soil.[3]
Structure
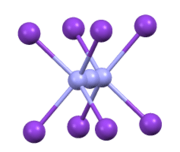
KN3, RbN3, CsN3, and TlN3 adopt the same structures. They crystallize in a tetragonal habit.[4] The azide is bound to eight cations in an eclipsed orientation. The cations are bound to eight terminal N centers.[5]
Synthesis and reactions
KN3 is prepared by treating potassium carbonate with hydrazoic acid, which is generated in situ.[6] In contrast, the analogous sodium azide is prepared (industrially) by the "Wislicenus process," which proceeds via the reaction sodium amide with nitrous oxide.[7]
Upon heating or upon irradiation with ultraviolet light, it decomposes into potassium metal and nitrogen gas.[8] The decomposition temperatures of the alkali metal azides are: NaN3 (275 °C), KN3 (355 °C), RbN3 (395 °C), CsN3 (390 °C).[9]
Health hazards
Like sodium azide, potassium azide is very toxic. The TLV of the related sodium azide is 0.07 ppm. The toxicity of azides arise from their ability to inhibit cytochrome c oxidase.[7]
References
- 1 2 3 Dale L. Perry; Sidney L. Phillips (1995). Handbook of inorganic compounds. CRC Press. p. 301. ISBN 0-8493-8671-3.
- ↑ http://chem.sis.nlm.nih.gov/chemidplus/rn/20762-60-1
- ↑ T. D. Hughes; L. F. Welch (1970). "Potassium Azide as a Nitrification Inhibitor". Agronomy Journal. American Society of Agronomy. 62: 595–599. doi:10.2134/agronj1970.00021962006200050013x.
- ↑ Khilji, M. Y.; Sherman, W. F.; Wilkinson, G. R. (1982). "Variable temperature and pressure Raman spectra of potassium azide KN
3". Journal of Raman Spectroscopy. 12 (3): 300–303. Bibcode:1982JRSp...12..300K. doi:10.1002/jrs.1250120319. - ↑ Ulrich Müller "Verfeinerung der Kristallstrukturen von KN3, RbN3, CsN3 und TIN3" Zeitschrift für anorganische und allgemeine Chemie 1972, Volume 392, 159–166. doi:10.1002/zaac.19723920207
- ↑ P. W. Schenk "Alkali Azides from Carbonates" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 475.
- 1 2 Horst H. Jobelius, Hans-Dieter Scharff "Hydrazoic Acid and Azides" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a13_193
- ↑ Tompkins, F. C.; Young, D. A. (1982). "The Photochemical and Thermal Formation of Colour Centres in Potassium Azide Crystals". Proceedings of the Royal Society of London. Series A, Mathematical and Physical Sciences. 236 (1204): 10–23.
- ↑ E. Dönges "Alkali Metals" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 475.
