Potassium ferrioxalate
_trihydrate_in_vial.jpg) | |
 | |
| Names | |
|---|---|
| IUPAC name
Potassium iron(III) oxalate | |
| Other names
potassium ferrioxalate potassium trisoxalatoferrate(III) | |
| Identifiers | |
| 5936-11-8 5936-11-8 (trihydrate) | |
| ECHA InfoCard | 100.035.398 |
| Properties | |
| K3[Fe(C2O4)3] (anhydrous) K3[Fe(C2O4)3]·3H2O (trihydrate) | |
| Molar mass | 437.20 g/mol (anhydrous) 491.25 g/mol (trihydrate) |
| Appearance | emerald green hydrated crystals |
| Density | 2.13 g/cm3 |
| Melting point | 230 °C (446 °F; 503 K) |
| Structure | |
| octahedral | |
| 0 D | |
| Hazards | |
| Main hazards | Corrosive. Eye, respiratory and skin irritant. |
| R-phrases | R20, R21, R22, R34, R36/37/38 |
| Related compounds | |
| Other anions |
Sodium ferrioxalate |
| Related compounds |
Iron(II) oxalate Iron(III) oxalate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Potassium ferrioxalate, also known as potassium trisoxalatoferrate(III), is a chemical compound with the formula K3[Fe(C2O4)3], where iron is in the +3 oxidation state. It is an octahedral transition metal complex in which three bidentate oxalate ions are bound to an iron center. Potassium acts as a counterion, balancing the −3 charge of the complex. Crystals of the trihydrated form of the complex, K3[Fe(C2O4)3]·3H2O, are emerald green in color. In solution, the salt dissociates to give the ferrioxalate anion, [Fe(C2O4)3]3−, which appears fluorescent green in color. Potassium ferrioxalate is often used in chemical actinometry, i.e. the measure of light flux.
Preparation
_trihydrate.jpg)
The complex can be synthesized by the reaction between iron(III) sulfate, barium oxalate and potassium oxalate:[1]
- Fe2(SO4)3 + 3 BaC2O4 + 3 K2C2O4 → 2 K3[Fe(C2O4)3] + 3 BaSO4
The reactants are combined in water, the solid precipitate of BaSO4 is removed, and the green trihydrate crystallizes from the cooled solution.
Another common synthesis is reacting aqueous iron(III) chloride hexahydrate and potassium oxalate monohydrate. Filter the solid using filter paper and a Büchner funnel and recrystallize the product to remove impurities.[2]
- FeCl3·6H2O + 3 K2C2O4•H2O → K3[Fe(C2O4)3]·3H2O + 3 KCl + 6 H2O
Structure
The ferrioxalate complex has D3 molecular symmetry, within which the iron center is octahedral. The six Fe–O bond distances all close to 2.0 Å[3] which indicates that the Fe(III) is high spin; as the low spin complex would display Jahn–Teller distortions. The ammonium and mixed sodium-potassium salts are isomorphous, as are related complexes with Al3+, Cr3+, and V3+.
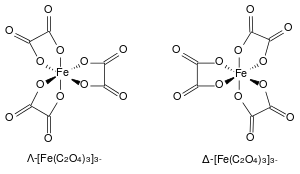
The ferrioxalate complex displays helical chirality as it can form two non-superimposable geometries. In accordance with the IUPAC convention, the isomer with the left-handed screw axis is assigned the Greek symbol Λ (lambda). Its mirror image with the right-handed screw axis is given the Greek symbol Δ (delta).[4]
Photoreduction
In solution, the ferrioxalate complex undergoes photoreduction. In this process, the complex absorbs a photon of light and subsequently decomposes to form Fe(C
2O
4)2−
2 and CO2. The iron centre is reduced (gains an electron) from the +3 to the +2 oxidation state while an oxalate ion is oxidised to carbon dioxide:
- 2 [Fe(C2O4)3]3− + hv → 2 [Fe(C2O4)2]2− + 2 CO2 + C
2O2−
4[5]
See also
A number of other iron oxalates are known
References
- ↑ Bailar, John C.; Jones, Eldon M. (1939). "Trioxalato Salts (Trioxalatoaluminiate, -ferriate, -chromiate, and -cobaltiate)". Inorg. Synth. 1: 35–38. doi:10.1002/9780470132326.ch13.
- ↑ Performed by Benjamin J. Abram, OSU lab manual, Dr. Richard Nafshun
- ↑ Junk, Peter C. (2005). "Supramolecular interactions in the X-ray crystal structure of potassium tris(oxalato)ferrate(III) trihydrate". J. Coord. Chem. 58 (4): 355–361. doi:10.1080/00958970512331334250.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0-08-037941-9.
- ↑ Pozdnyakov, Ivan P.; Kel, Oksana V.; Plyusnin, Victor F.; Grivin, Vyacheslav P.; Bazhin, Nikolai M. (2008). "New Insight into Photochemistry of Ferrioxalate". J. Phys. Chem. A. 112 (36): 8316–8322. doi:10.1021/jp8040583.