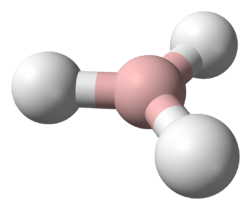
Hydrazoic acid
 | |
| | |
 | |
| Names | |
|---|---|
| IUPAC name
Hydrogen azide | |
| Identifiers | |
| 7782-79-8 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:29449 |
| ChEMBL | ChEMBL186537 |
| ChemSpider | 22937 |
| ECHA InfoCard | 100.029.059 |
| PubChem | 24530 |
| |
| |
| Properties | |
| HN3 | |
| Molar mass | 43.03 g/mol |
| Appearance | colorless, highly volatile liquid |
| Density | 1.09 g/cm3 |
| Melting point | −80 °C (−112 °F; 193 K) |
| Boiling point | 37 °C (99 °F; 310 K) |
| highly soluble | |
| Solubility | soluble in alkali, alcohol, ether |
| Acidity (pKa) | 4.6 [1] |
| Structure | |
| approximately linear | |
| Hazards | |
| Main hazards | Highly toxic, explosive, reactive |
| R-phrases | R3, R27/28 |
| S-phrases | S33, S36/37, S38 |
| NFPA 704 | |
| Related compounds | |
| Other cations |
Sodium azide |
| Ammonia Hydrazine | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Hydrazoic acid, also known as hydrogen azide or azoimide,[2] is a compound with the chemical formula HN3.[3] It is a colorless, volatile, and explosive liquid at room temperature and pressure. It is a compound of nitrogen and hydrogen, and is therefore a pnictogen hydride. It was first isolated in 1890 by Theodor Curtius.[4] The acid has few applications, but its conjugate base, the azide ion, is useful in specialized processes.
Hydrazoic acid is soluble in water. Undiluted hydrazoic acid is dangerously explosive[5] with a standard enthalpy of formation ΔfHo (l, 298K) = +264 kJmol−1).[6] When dilute, the gas and aqueous solutions (<10%) can be safely handled.
Production
The acid is usually formed by acidification of an azide salt like sodium azide. Normally solutions of sodium azide in water contain trace quantities of hydrazoic acid in equilibrium with the azide salt, but introduction of a stronger acid can convert the primary species in solution to hydrazoic acid. The pure acid may be subsequently obtained by fractional distillation as an extremely explosive colorless liquid with an unpleasant smell.
- NaN3 + HCl → HN3 + NaCl
Its aqueous solution can also be prepared by treatment of barium azide solution with dilute sulfuric acid, filtering the insoluble barium sulfate.[7]
It was originally prepared by the reaction of aqueous hydrazine with nitrous acid.
- N2H5+ + HNO2 → HN3 + H+ + 2 H2O
Other oxidizing agents, such as hydrogen peroxide, NOCl, NCl3 or nitric acid, can also be used.[8]
Reactions
In its properties hydrazoic acid shows some analogy to the halogen acids, since it forms poorly soluble (in water) lead, silver and mercury(I) salts. The metallic salts all crystallize in the anhydrous form and decompose on heating, leaving a residue of the pure metal. It is a weak acid (pKa = 4.75.[6]) Its heavy metal salts are explosive and readily interact with the alkyl iodides. Azides of heavier alkali metals (excluding lithium) or alkaline earth metals are not explosive, but decompose in a more controlled way upon heating, releasing spectroscopically-pure N
2 gas.[9] Solutions of hydrazoic acid dissolve many metals (e.g. zinc, iron) with liberation of hydrogen and formation of salts, which are called azides (formerly also called azoimides or hydrazoates).
Dissolution in the strongest acids produces explosive salts containing the H
2N=N=N+
ion, for example:[9]
- HN=N=N + HSbCl
6 → [H
2N=N=N]+
[SbCl
6]−
The ion H
2N=N=N+
is isoelectronic to diazomethane.
The decomposition of hydrazoic acid, triggered by shock, friction, spark, etc. goes as follows:
- 2HN
3 → H
2 + 3N
2
Toxicity
Hydrazoic acid is volatile and highly toxic. It has a pungent smell and its vapor can cause violent headaches. The compound acts as a non-cumulative poison.
Applications
2-Furonitrile, a pharmaceutical intermediate and potential artificial sweetening agent has been prepared in good yield by treating furfural with a mixture of hydrazoic acid (HN3) and perchloric acid in the presence of magnesium perchlorate in the benzene solution at 35 °C.[10][11]
The all gas-phase iodine laser (AGIL) mixes gaseous hydrazoic acid with chlorine to produce excited nitrogen chloride, which is then used to cause iodine to lase; this avoids the liquid chemistry requirements of COIL lasers.
References
- ↑ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0-07-049439-8
- ↑
 Chisholm, Hugh, ed. (1911). "Azoimide". Encyclopædia Britannica. 3 (11th ed.). Cambridge University Press.
Chisholm, Hugh, ed. (1911). "Azoimide". Encyclopædia Britannica. 3 (11th ed.). Cambridge University Press. - ↑ Dictionary of Inorganic and Organometallic Compounds. Chapman & Hall.
- ↑ Curtius, Theodor (1890). "Ueber Stickstoffwasserstoffsäure (Azoimid) N3H" [On hydrazoic acid (azoimide) N3H]. Berichte der deutschen chemischen Gesellschaft. 23 (2): 3023–3033. doi:10.1002/cber.189002302232.
- ↑ Furman, David; Dubnikova, Faina; van Duin, Adri C. T.; Zeiri, Yehuda; Kosloff, Ronnie (2016-03-10). "Reactive Force Field for Liquid Hydrazoic Acid with Applications to Detonation Chemistry". The Journal of Physical Chemistry C. 120 (9): 4744–4752. doi:10.1021/acs.jpcc.5b10812. ISSN 1932-7447.
- 1 2 Catherine E. Housecroft; Alan G. Sharpe (2008). "Chapter 15: The group 15 elements". Inorganic Chemistry, 3rd Edition. Pearson. p. 449. ISBN 978-0-13-175553-6.
- ↑ L . F. Audrieth, C. F. Gibbs Hydrogen Azide in Aqueous and Ethereal Solution" Inorganic Syntheses 1939, vol. 1, pp. 71-79.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 432. ISBN 0-08-037941-9.
- 1 2 Egon Wiberg; Nils Wiberg; Arnold Frederick Holleman (2001). "The Nitrogen Group". Inorganic chemistry. Academic Press. p. 625. ISBN 0-12-352651-5.
- ↑ P. A. Pavlov; Kul'nevich, V. G. (1986). "Synthesis of 5-substituted furannitriles and their reaction with hydrazine". Khimiya Geterotsiklicheskikh Soedinenii. 2: 181–186.
- ↑ B. Bandgar; Makone, S. (2006). "Organic reactions in water. Transformation of aldehydes to nitriles using NBS under mild conditions". Synthetic Communications. 36 (10): 1347–1352. doi:10.1080/00397910500522009.