SIR proteins
Silent Information Regulator (SIR) proteins are involved in regulating gene expression and some SIR family members are conserved from yeast to humans. SIR proteins organize heterochromatin at telomeres,[1] rDNA[2] and at silent loci including, in yeast, the hidden mating type loci.[3][4] SIR family genes encode catalytic and non-catalytic proteins that are involved in de-acetylation of histone tails and the subsequent condensation of chromatin around a SIR protein scaffold.[5]
History
SIR proteins have been identified in many screens, and have historically been known as SIR[3] (silent information regulator), MAR[6] (mating-type regulator), STE[7] (sterile), CMT[8] (change of mating type) or SSP[9] (sterile suppressor) according to which screen led to their identification. Ultimately, the name SIR had the most staying power, because it most accurately describes the function of the encoded proteins.
One of the early yeast screens to identify SIR genes was performed by Anita Hopper and Benjamin Hall, who screened with mutagenesis for alleles that allow sporulation in a normally sporulation-deficient heterothallic α/α (ho/ho MATα/MATα). Their screen identified a mutation in a novel gene that was not linked to HO that allowed the α/α diploid to sporulate, as if it were an α/a diploid, and inferred that the mutation affected a change in mating type by an HO-independent mechanism.[8] Later, it was discovered at the CMT allele identified by Hopper & Hall did not cause a mating type conversion at the MAT locus, but rather allowed the expression of cryptic mating type genes that are silenced in wild-type yeast.[4] In their paper clarifying the mechanism of the CMT mutation, Haber and George acknowledge the contribution of Amar Klar, who presented his MAR mutant strains that had similar properties as the CMT mutants at the Cold Spring Harbor Laboratory yeast genetics meeting, which led Haber and George to consider the hypotheses that the cmt mutants may act by de-repressing silent information.[10]
In the same year that Haber & George demonstrated that the cmt mutant restores sporulation by de-repressing hidden mating type loci, two other groups published screens for genes involved in the regulation of silent mating type cassettes.[6] The first study, performed by Amar Klar, Seymour Fogel and Kathy Macleod, identified a mutation in a spontaneous a/a diploid that caused the products of sporulation to be haploids with an apparent diploid phenotype, as assayed by ability to mate.[6] The authors reasoned that the mutation caused the de-repression of then-recently appreciated silent mating type loci HMa and HMα, which would allow an a/a diploid to sporulate and would cause haploid segregants inheriting the mutant allele to behave as a/α diploids despite being haploid.[6] The authors named the mutation MAR for its apparent role in mating type regulation, and were able to map the mutation to chromosome IV, and determined that it was located 27.3 cM from a commonly used trp1 marker.[6]
A few months later, Jasper Rine and Ira Herskowitz published a different screen for genes that affect the ability of yeast to mate, and ultimate discovered the gene family that they called SIR, a name that remains in the modern parlance.[3] Unlike the Klar et al. screen that identified a mutant by its inability to mate, Rine & Herskowitz took a more directed approach towards discovering factors responsible for mating type silencing. Specifically, Rine & Herskowitz reasoned that a haploid yeast cell with a recessive mutation in matα1 could be complemented if the silent copy of MATα were de-repressed. Starting in a ho matα1 haploid strain, Rine & Herskowitz screened mutants arising from mutagenesis and identified five mutants that restored a MATα phenotype in matα cells, but were not linked to the MAT locus and did not cause a gene conversion between the HMα locus and matα.[3] These mutants, they reasoned, were specifically defective in silencing the cryptic mating type genes.
Eventually, all of the mutants resulting from the original Hopper & Hall screen as well as the later Rine & Herskowitz screen and the Klar et al. screen were characterized and mapped, and it was shown that the causative genes were the same.[11] In fact, the genes that are now referred to as SIR1-4 have at one time been referred to as MAR, CMT or STE according to the screen that identified the mutants.
Although Klar, Hartwell and Hopper identified mutations in SIR genes and applied other names to the genes before Rine performed his screen, the SIR name was eventually adopted because Rine eventually identified the most complete set of functionally related genes (SIR1-4), and because the work by Rine and Herskowitz most accurately described the function of the SIR family genes.[11] Later it would be shown that in yeast and in higher organisms, SIR proteins are important for transcriptional regulation of many chromatin domains.
Molecular mechanism
In budding yeast, SIR proteins are found at the silent mating type loci, telomeres, and at the rDNA locus. At the silent mating type loci and at the telomeres, SIR proteins participate in transcriptional silencing of genes within their domain of localization. At the rDNA locus, SIR proteins are thought to primarily be important for repressing recombination between rDNA repeats rather than for suppressing transcription.[12]
Transcriptional silencing in budding yeast
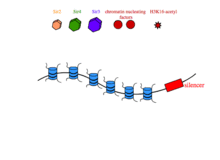
In transcriptional silencing, SIR2,3,4 are required in stoichiometric amounts to silence genes in cis. In yeast, SIR proteins bind sites on nucleosome tails and form a multimeric compound of SIR2,3,4 that condenses chromatin and is thought to physically occlude promoters in the silenced interval, preventing their interaction with transcription machinery.[12] The establishment of SIR-reppressed heterochromatin domains is a complicated process that involves different subsets of proteins and regulatory proteins depending on the locus in the genome.[12] At the silent mating type loci and at yeast telomeres, the transcription factors Abf1 (ARS binding factor) and Rap1 (repressor-activator protein) associate with specific nucleotide sequences in the silencers that flank heterochromatic regions.[13] Rap1 contains a Sir3-binding domain that recruits SIR3 to the silencers.[14] Once at the silencers, Sir3 recruits Sir4-Sir2 dimers to the chromatin nucleation site. Sir2 then deacetylates histone H3 and H4 tails, and free Sir3 binds the now-deacetylated lysine residues H4K16,79, and recruits additional Sir4-Sir2 dimers to promote the further spreading of the heterochromatin domain.[12]
Once it has spread to cover a genomic locus, the SIR2,3,4 effectively prevents transcription from the region it occupies, in a process that is thought to depend on the physical occlusion of DNA by SIR proteins. Recently, it has been shown that certain promoters are capable of directing transcription inside regions that are otherwise silenced by SIR proteins.[15] Specifically, if an inducible promoter is induced inside a silent chromatin domain, it can achieve ~200x increase in expression levels with little detectable change in covalent histone modifications.[15]
Conservation
SIR proteins are conserved from yeast to humans, and lend their name to a class of mammalian histone deacetylases (Sirtuins, homologs of Sir2). Sirtuins have been implicated in myriad human traits including Alzheimers and diabetes, and have been proposed to regulate of lifespan.
See also
References
- ↑ Palladino F; Laroche T; Gilson E; Axelrod A; Pillus L; Gasser SM (November 1993). "SIR3 and SIR4 proteins are required for the positioning and integrity of yeast telomeres". Cell. 75 (3): 543–55. doi:10.1016/0092-8674(93)90388-7. PMID 8221893.
- ↑ Smith JS; Boeke JD (January 1997). "An unusual form of transcriptional silencing in yeast ribosomal DNA". Genes & Development. 11 (2): 241–54. doi:10.1101/gad.11.2.241. PMID 9009206.
- 1 2 3 4 Rine J; Strathern JN; Hicks JB; Herskowitz I (December 1979). "A suppressor of mating-type locus mutations in Saccharomyces cerevisiae: evidence for and identification of cryptic mating-type loci". Genetics. 93 (4): 877–901. PMC 1214119
 . PMID 397913.
. PMID 397913. - 1 2 Haber JE; George JP (September 1979). "A mutation that permits the expression of normally silent copies of mating-type information in Saccharomyces cerevisiae". Genetics. 93 (1): 13–35. PMC 1217820
 . PMID 16118901.
. PMID 16118901. - ↑ Thurtle DM; Rine J (February 2014). "The molecular topography of silenced chromatin in Saccharomyces cerevisiae". Genes & Development. 28 (3): 245–58. doi:10.1101/gad.230532.113. PMC 3923967
 . PMID 24493645.
. PMID 24493645. - 1 2 3 4 5 Klar AJ; Fogel S; Macleod K (September 1979). "MAR1-a Regulator of the HMa and HMalpha Loci in SACCHAROMYCES CEREVISIAE". Genetics. 93 (1): 37–50. PMC 1217836
 . PMID 17248968.
. PMID 17248968. - ↑ Hartwell LH (June 1980). "Mutants of Saccharomyces cerevisiae unresponsive to cell division control by polypeptide mating hormone". The Journal of Cell Biology. 85 (3): 811–22. doi:10.1083/jcb.85.3.811. PMC 2111434
 . PMID 6993497.
. PMID 6993497. - 1 2 Hopper AK; Hall BD (May 1975). "Mutation of a heterothallic strain to homothallism". Genetics. 80 (1): 77–85. PMC 1213321
 . PMID 1093938.
. PMID 1093938. - ↑ Hicks, James Bruce (1975). Interconversion of Mating Types in Yeast (PhD Thesis). University of Oregon. OCLC 276853119.
- ↑ Klar AJ (October 2010). "The yeast mating-type switching mechanism: a memoir". Genetics. 186 (2): 443–9. doi:10.1534/genetics.110.122531. PMC 2942867
 . PMID 20940334.
. PMID 20940334. - 1 2 Ivy JM; Hicks JB; Klar AJ (December 1985). "Map positions of yeast genes SIR1, SIR3 and SIR4". Genetics. 111 (4): 735–44. PMC 1202668
 . PMID 3905505.
. PMID 3905505. - 1 2 3 4 Kueng S; Oppikofer M; Gasser SM (2013). "SIR proteins and the assembly of silent chromatin in budding yeast". Annual Review of Genetics. 47: 275–306. doi:10.1146/annurev-genet-021313-173730. PMID 24016189.
- ↑ McNally FJ; Rine J (November 1991). "A synthetic silencer mediates SIR-dependent functions in Saccharomyces cerevisiae". Molecular and Cellular Biology. 11 (11): 5648–59. PMC 361936
 . PMID 1922068.
. PMID 1922068. - ↑ Moretti P; Freeman K; Coodly L; Shore D (October 1994). "Evidence that a complex of SIR proteins interacts with the silencer and telomere-binding protein RAP1". Genes & Development. 8 (19): 2257–69. doi:10.1101/gad.8.19.2257. PMID 7958893.
- 1 2 Zhang H; Gao L; Anandhakumar J; Gross DS (April 2014). "Uncoupling transcription from covalent histone modification". PLoS Genetics. 10 (4): e1004202. doi:10.1371/journal.pgen.1004202. PMC 3983032
 . PMID 24722509.
. PMID 24722509.