Sirtuin
| Sir2 family | |||||||||
|---|---|---|---|---|---|---|---|---|---|
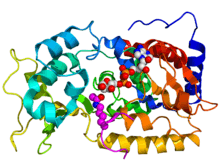 Crystallographic structure of yeast sir2 (rainbow colored cartoon, N-terminus = blue, C-terminus = red) complexed with ADP (space-filling model, carbon = white, oxygen = red, nitrogen = blue, phosphorus = orange) and a histone H4 peptide (magenta) containing an acylated lysine residue (displayed as spheres).[1] | |||||||||
| Identifiers | |||||||||
| Symbol | SIR2 | ||||||||
| Pfam | PF02146 | ||||||||
| Pfam clan | CL0085 | ||||||||
| InterPro | IPR003000 | ||||||||
| PROSITE | PS50305 | ||||||||
| SCOP | 1j8f | ||||||||
| SUPERFAMILY | 1j8f | ||||||||
| |||||||||
Sirtuin or Sir2 proteins are a class of proteins that possess either mono-ADP-ribosyltransferase, or deacylase activity, including deacetylase, desuccinylase, demalonylase, demyristoylase and depalmitoylase activity.[2][3][4][5][6] Sirtuins regulate important biological pathways in bacteria, archaea and eukaryotes. The name Sir2 comes from the yeast gene 'silent mating-type information regulation 2',[7] the gene responsible for cellular regulation in yeast.
Sirtuins have been implicated in influencing a wide range of cellular processes like aging, transcription, apoptosis, inflammation[8] and stress resistance, as well as energy efficiency and alertness during low-calorie situations.[9] Sirtuins can also control circadian clocks and mitochondrial biogenesis.
Yeast Sir2 and some, but not all, sirtuins are protein deacetylases. Unlike other known protein deacetylases, which simply hydrolyze acetyl-lysine residues, the sirtuin-mediated deacetylation reaction couples lysine deacetylation to NAD hydrolysis. This hydrolysis yields O-acetyl-ADP-ribose, the deacetylated substrate and nicotinamide, itself an inhibitor of sirtuin activity. The dependence of sirtuins on NAD links their enzymatic activity directly to the energy status of the cell via the cellular NAD:NADH ratio, the absolute levels of NAD, NADH or nicotinamide or a combination of these variables.
Species distribution
Whereas bacteria and archaea encode either one or two sirtuins, eukaryotes encode several sirtuins in their genomes. In yeast, roundworms, and fruitflies, sir2 is the name of the sirtuin-type protein.[10] This research started in 1991 by Leonard Guarente of MIT.[11][12] Mammals possess seven sirtuins (SIRT1-7) that occupy different subcellular compartments such as the nucleus (SIRT1, -2, -6, -7), cytoplasm (SIRT1 and SIRT2) and the mitochondria (SIRT3, -4 and -5).
Types
The first sirtuin was identified in yeast (a lower eukaryote) and named sir2. In more complex mammals, there are seven known enzymes that act in cellular regulation, as sir2 does in yeast. These genes are designated as belonging to different classes (I-IV), depending on their amino acid sequence structure.[13][14] Several Gram positive prokaryotes as well as the Gram negative hyperthermophilic bacterium Thermotoga maritima possess sirtuins that are intermediate in sequence between classes and these are placed in the "undifferentiated" or "U" class.[13] In addition, several Gram positive bacteria, including Staphylococcus aureus and Streptococcus pyogenes, as well as several fungi carry macrodomain-linked sirtuins (termed "class M" sirtuins).[6] Most notable, the latter have an altered catalytic residue, which make them exclusive ADP-ribosyl transferases.
| Class | Subclass | Species | Intracellular location |
Activity | Function | |||
|---|---|---|---|---|---|---|---|---|
| Bacteria | Yeast | Mouse | Human | |||||
| I | a | Sir2 or Sir2p, Hst1 or Hst1p | Sirt1 | SIRT1 | nucleus, cytoplasm | deacetylase | metabolism inflammation | |
| b | Hst2 or Hst2p | Sirt2 | SIRT2 | cytoplasm | deacetylase | cell cycle, tumorigenesis | ||
| Sirt3 | SIRT3 | nucleus and mitochondria | deacetylase | metabolism | ||||
| c | Hst3 or Hst3p, Hst4 or Hst4p | |||||||
| II | Sirt4 | SIRT4 | mitochondria | ADP-ribosyl transferase | insulin secretion | |||
| III | Sirt5 | SIRT5 | mitochondria | demalonylase, desuccinylase and deacetylase | ammonia detoxification | |||
| IV | a | Sirt6 | SIRT6 | nucleus | Demyristoylase, depalmitoylase, ADP-ribosyl transferase and deacetylase | DNA repair, metabolism, TNF secretion | ||
| b | Sirt7 | SIRT7 | nucleolus | deacetylase | rRNA transcription | |||
| U | cobB[15] | regulation of acetyl-CoA synthetase[16] | metabolism | |||||
| M | SirTM[6] | ADP-ribosyl transferase | ROS detoxification | |||||
Sirtuin list based on North/Verdin diagram.[17]
Clinical significance
Sirtuin activity is inhibited by nicotinamide, which binds to a specific receptor site,[18] so it is thought that drugs that interfere with this binding should increase sirtuin activity. Development of new agents that would specifically block the nicotinamide-binding site could provide an avenue for development of newer agents to treat degenerative diseases such as cancer, diabetes, atherosclerosis, and gout.[19][20]
Diabetes
Sirtuins have been proposed as a therapeutic target for type II diabetes mellitus.[21]
Aging
Preliminary studies with resveratrol, a possible SIRT1 activator, have led some scientists to speculate that resveratrol may extend lifespan.[22] Further experiments conducted by Rafael de Cabo et al. showed that resveratrol-mimicking drugs such as SRT1720 could extend the lifespan of obese mice by 44%.[23] Comparable molecules are now undergoing clinical trials in humans.
Cell culture research into the behaviour of the human sirtuin SIRT1 shows that it behaves like the yeast sirtuin Sir2: SIRT2 assists in the repair of DNA and regulates genes that undergo altered expression with age.[24] Adding resveratrol to the diet of mice inhibit gene expression profiles associated with muscle aging and age-related cardiac dysfunction.[25]
A study performed on transgenic mice overexpressing SIRT6, showed an increased lifespan of about 15% in males. The transgenic males displayed lower serum levels of insulin-like growth factor 1 (IGF1) and changes in its metabolism, which may have contributed to the increased lifespan.[26]
See also
- Ageing
- Sir2
- Resveratrol
- Biological immortality
- Caloric restriction
- Trichostatin A
- Histone deacetylases or HDACs
References
- ↑ PDB: 1szd; Zhao K, Harshaw R, Chai X, Marmorstein R (June 2004). "Structural basis for nicotinamide cleavage and ADP-ribose transfer by NAD(+)-dependent Sir2 histone/protein deacetylases". Proc. Natl. Acad. Sci. U.S.A. 101 (23): 8563–8. doi:10.1073/pnas.0401057101. PMC 423234
 . PMID 15150415.
. PMID 15150415. - ↑ North BJ, Verdin E (2004). "Sirtuins: Sir2-related NAD-dependent protein deacetylases". Genome Biol. 5 (5): 224. doi:10.1186/gb-2004-5-5-224. PMC 416462
 . PMID 15128440.
. PMID 15128440. - ↑ Yamamoto H, Schoonjans K, Auwerx J (August 2007). "Sirtuin functions in health and disease". Mol. Endocrinol. 21 (8): 1745–55. doi:10.1210/me.2007-0079. PMID 17456799.
- ↑ Du J, Zhou Y, Su X, Yu JJ, Khan S, Jiang H, Kim J, Woo J, Kim JH, Choi BH, He B, Chen W, Zhang S, Cerione RA, Auwerx J, Hao Q, Lin H (2011). "Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase.". Science. 334 (6057): 806–809. doi:10.1126/science.1207861. PMC 3217313
 . PMID 22076378.
. PMID 22076378. - ↑ Jiang H, Khan S, Wang Y, Charron G, He B, Sebastian C, Du J, Kim R, Ge E, Mostoslavsky R, Hang HC, Hao Q, Lin H (2013). "SIRT6 regulates TNF-α secretion through hydrolysis of long-chain fatty acyl lysine.". Nature. 496 (7443): 110–113. doi:10.1038/nature12038. PMC 3635073
 . PMID 23552949.
. PMID 23552949. - 1 2 3 Rack, Johannes Gregor Matthias; Morra, Rosa; Barkauskaite, Eva; Kraehenbuehl, Rolf; Ariza, Antonio; Qu, Yue; Ortmayer, Mary; Leidecker, Orsolya; Cameron, David R. (2015-07-16). "Identification of a Class of Protein ADP-Ribosylating Sirtuins in Microbial Pathogens". Molecular Cell. 59 (2): 309–320. doi:10.1016/j.molcel.2015.06.013. ISSN 1097-4164. PMC 4518038
 . PMID 26166706.
. PMID 26166706. - ↑ EntrezGene 23410
- ↑ Preyat N, Leo O (2013). "Sirtuin deacylases: a molecular link between metabolism and immunity.". J. Leuk. Biol. 93 (5): 669–680. doi:10.1189/jlb.1112557. PMID 23325925.
- ↑ Satoh A, Brace CS, Ben-Josef G, West T, Wozniak DF, Holtzman DM, Herzog ED, Imai S (2010). "SIRT1 Promotes the Central Adaptive Response to Diet Restriction through Activation of the Dorsomedial and Lateral Nuclei of the Hypothalamus.". Journal of Neuroscience. 30 (30): 10220–32. doi:10.1523/JNEUROSCI.1385-10.2010. PMC 2922851
 . PMID 20668205.
. PMID 20668205. - ↑ Blander G, Guarente L (2004). "The Sir2 family of protein deacetylases". Annu. Rev. Biochem. 73 (1): 417–35. doi:10.1146/annurev.biochem.73.011303.073651. PMID 15189148.
- ↑ Wade N (2006-11-08). "The quest for a way around aging". Health & Science. International Herald Tribune. Retrieved 2008-11-30.
- ↑ "MIT researchers uncover new information about anti-aging gene". Massachusetts Institute of Technology, News Office. 2000-02-16. Retrieved 2008-11-30.
- 1 2 Frye R (2000). "Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins". Biochem Biophys Res Commun. 273 (2): 793–8. doi:10.1006/bbrc.2000.3000. PMID 10873683.
- ↑ Dryden S, Nahhas F, Nowak J, Goustin A, Tainsky M (2003). "Role for human SIRT2 NAD-dependent deacetylase activity in control of mitotic exit in the cell cycle". Mol Cell Biol. 23 (9): 3173–85. doi:10.1128/MCB.23.9.3173-3185.2003. PMC 153197
 . PMID 12697818.
. PMID 12697818. - ↑ Zhao K, Chai X, Marmorstein R (March 2004). "Structure and substrate binding properties of cobB, a Sir2 homolog protein deacetylase from Escherichia coli". J. Mol. Biol. 337 (3): 731–41. doi:10.1016/j.jmb.2004.01.060. PMID 15019790.
- ↑ Schwer B, Verdin E (February 2008). "Conserved metabolic regulatory functions of sirtuins". Cell Metab. 7 (2): 104–12. doi:10.1016/j.cmet.2007.11.006. PMID 18249170.
- ↑ North B, Verdin E (2004). "Sirtuins: Sir2-related NAD-dependent protein deacetylases". Genome Biol. 5 (5): 224. doi:10.1186/gb-2004-5-5-224. PMC 416462
 . PMID 15128440.
. PMID 15128440. - ↑ Avalos JL, Bever KM, Wolberger C (March 2005). "Mechanism of sirtuin inhibition by nicotinamide: altering the NAD(+) cosubstrate specificity of a Sir2 enzyme". Mol. Cell. 17 (6): 855–68. doi:10.1016/j.molcel.2005.02.022. PMID 15780941.
- ↑ Adams JD Jr; Klaidman LK (2008). "Sirtuins, Nicotinamide and Aging: A Critical Review" (PDF). Letters in Drug Design & Discovery. 4 (1): 44–48. doi:10.2174/157018007778992892.
- ↑ Taylor DM, Maxwell MM, Luthi-Carter R, Kazantsev AG (September 2008). "Biological and Potential Therapeutic Roles of Sirtuin Deacetylases". Cell. Mol. Life Sci. 65 (24): 4000–18. doi:10.1007/s00018-008-8357-y. PMID 18820996.
- ↑ Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH (November 2007). "Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes". Nature. 450 (7170): 712–6. doi:10.1038/nature06261. PMC 2753457
 . PMID 18046409.
. PMID 18046409. - ↑ Wade N (2008-06-04). "New Hints Seen That Red Wine May Slow Aging". NYTimes.com. Retrieved 2008-11-30.
- ↑ Wade N (2011-08-18). "Longer Lives for Obese Mice, With Hope for Humans of All Sizes". NYTimes.com. Retrieved 2012-05-13.
- ↑ Oberdoerffer P, Michan S, McVay M, Mostoslavsky R, Vann J, Park SK, Hartlerode A, Stegmuller J, Hafner A, Loerch P, Wright SM, Mills KD, Bonni A, Yankner BA, Scully R, Prolla TA, Alt FW, Sinclair DA (November 2008). "SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging". Cell. 135 (5): 907–18. doi:10.1016/j.cell.2008.10.025. PMC 2853975
 . PMID 19041753.
. PMID 19041753. - ↑ Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, Wang Y, Raederstorff D, Morrow JD, Leeuwenburgh C, Allison DB, Saupe KW, Cartee GD, Weindruch R, Prolla TA (2008). Tomé D, ed. "A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice". PLoS ONE. 3 (6): e2264. doi:10.1371/journal.pone.0002264. PMC 2386967
 . PMID 18523577.
. PMID 18523577. - ↑ Kanfi, Yariv; Naiman, Shoshana; Amir, Gail; Peshti, Victoria; Zinman, Guy; Nahum, Liat; Bar-Joseph, Ziv; Cohen, Haim Y. (2012). "The sirtuin SIRT6 regulates lifespan in male mice". Nature. 483 (7388): 218–21. doi:10.1038/nature10815. ISSN 0028-0836. PMID 22367546.
External links
- Sirtuins at the US National Library of Medicine Medical Subject Headings (MeSH)
- Rice J (2008-11-26). "How Cells Age: Parallels between mice and yeast uncover a potentially universal aging mechanism". Technology Review. Massachusetts Institute of Technology. Retrieved 2008-12-20.