Scrapie

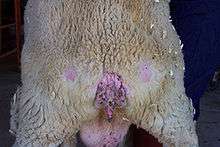
Scrapie is a fatal, degenerative disease that affects the nervous systems of sheep and goats.[1] It is one of several transmissible spongiform encephalopathies (TSEs), which are related to bovine spongiform encephalopathy (BSE or "mad cow disease") and chronic wasting disease of deer. Like other spongiform encephalopathies, scrapie is caused by a prion.[2] Scrapie has been known since 1732, and does not appear to be transmissible to humans.[3][4]
The name scrapie is derived from one of the clinical signs of the condition, wherein affected animals will compulsively scrape off their fleeces against rocks, trees, or fences. The disease apparently causes an itching sensation in the animals. Other clinical signs include excessive lip smacking, altered gaits, and convulsive collapse.[5]
Scrapie is infectious and transmissible among conspecifics, so one of the most common ways to contain it (since it is incurable) is to quarantine and destroy those affected. However, scrapie tends to persist in flocks and can also arise apparently spontaneously in flocks that have not previously had cases of the disease. The mechanism of transmission between animals and other aspects of the biology of the disease are only poorly understood, and these are active areas of research. Recent studies suggest prions may be spread through urine and persist in the environment for decades.[6]
Scrapie usually affects sheep around three to five years of age. The potential for transmission at birth and from contact with placental tissues is apparent. No evidence indicates scrapie is infectious to humans.[7]
Uptake of prions
The protein enters through the intestines or through cuts in the skin. The prions cause normal proteins of the sheep to fold into the wrong shape. These proteins are gradually accumulated in the body, especially in nerve cells, which subsequently die. When the prions are absorbed through the intestines, they first appear in the lymph nodes, especially in Peyer's patches at the small intestine.[8]
An experiment has shown lambs risk being infected through milk from infected ewes,[9] but the lambs in the experiment also infected each other, making the risk of infection difficult to assess. The experiment did not continue long enough to show if the lambs developed symptoms, but merely that the prion was present in their bodies.
Clinical signs and diagnosis
Changes are mild at first; slight behavioural changes and an increase in chewing movements may occur. Ataxia and neurological signs then develop, and affected sheep struggle to keep up with the flock.[7]
Some sheep scratch excessively and show patches of wool loss and lesions on the skin. Scratching sheep over the rump area may lead to a nibbling reflex, which is characteristic for the condition.[7]
Signs of a chronic systemic disease appear later, with weight loss, anorexia, lethargy, and death.[7]
Post mortem examination is important for the diagnosis of scrapie. Histology of tissues shows accumulation of prions in the central nervous system, and immunohistochemical staining and ELISA can also be used to demonstrate the protein.
Treatment and preventive action
No treatment is available for affected sheep.[7]
A test performed by sampling a small amount of lymphatic tissue from the third eyelid is now available.[10]
In the United Kingdom, the government has put in place a National Scrapie Plan, which encourages breeding from sheep that are genetically more resistant to scrapie. This is intended to eventually reduce the incidence of the disease in the UK sheep population. Scrapie occurs in Europe and North America, but to date, Australia and New Zealand (both major sheep-producing countries) are scrapie-free.
Breeds such as Cheviot and Suffolk are more susceptible to scrapie than other breeds.[11] Specifically, this is determined by the genes coding for the naturally occurring prion proteins. The most resistant sheep have a double set of ARR alleles, while sheep with the VRQ allele are the most susceptible.[12] A simple blood test reveals the allele of the sheep, and many countries are actively breeding away the VRQ allele.
Out of fear of BSE, many European countries banned some traditional sheep or goat products made without removing the spinal cord, such as smalahove and smokie.[13]
In 2010, a team from New York described detection of PrPSc even when initially present at only one part in a hundred billion (10−11) in brain tissue. The method combines amplification with a novel technology called surround optical fiber immunoassay and some specific antibodies against PrPSc. The technique allowed detection of PrPSc after many fewer cycles of conversion than others have achieved, substantially reducing the possibility of artefacts, as well as speeding up the assay. The researchers also tested their method on blood samples from apparently healthy sheep that went on to develop scrapie. The animals' brains were analysed once any symptoms became apparent. They could therefore compare results from brain tissue and blood taken once the animals exhibited symptoms of the diseases, with blood obtained earlier in the animals' lives, and from uninfected animals. The results showed very clearly that PrPSc could be detected in the blood of animals long before the symptoms appeared. After further development and testing, this method could be of great value in surveillance as a blood- or urine-based screening test for scrapie.[14][15]
Transmission/exposure pathways
Various studies have indicated prions (PrPSC) that infect sheep and goats with the fatal transmissible encephalopathy known as scrapie, are able to persist in soil for years without losing their pathogenic activity.[16] Dissemination of prions into the environment can occur from several sources: mainly, infectious placenta or amniotic fluid of sheep and possibly environmental contamination by saliva or excrement.
Confirmatory testing for scrapie can only be achieved by applying immunohistochemistry of disease-associated prion protein (PrPSC) to tissues collected post mortem, including obex (a brainstem structure), retropharyngeal lymph node and palatine tonsil. A live animal diagnostic, not confirmatory, test was approved in 2008 for immunochemistry testing on rectal biopsy-derived lymphoid tissue by USDA.

Natural transmission of scrapie in the field seems to occur via the alimentary tract in the majority of cases, and scrapie-free sheep flocks can become infected on pastures where outbreaks of scrapie had been observed before. These findings point to a sustained contagion in the environment, notably in the soil.[17]
Prion concentration in birth fluids does not alter the infectivity of the prions. Naturally or experimentally infected does and ewes transmit the infection to the kids, even when placentas have little PrPSC. PrPSC is shed at a higher percentage in sheep placentas (52%-72%) than in goat placenta (5-10%) in study trials at the USDA Agricultural Research Service.[18]
Detectable PrPSC has been reported in the feces of sheep both in the terminal and the early preclinical stages of the disease, suggesting the prions are likely to be shed into the environment throughout the course of the disease. Several sources of prions in feces could be postulated, including environmental ingestion and swallowing infected saliva; however, the most likely source is shedding from the gut-associated lymphoid tissue. Ruminant animals have specialized Peyer's patches that, throughout the length of the ileum, amount to about 100,000 follicles, and all of these could be infected and shedding prions into the lumen.[19] Scrapie prions have been found in the Peyer's patches of naturally infected asymptomatic lambs as young as four months of age.
Exposure through contaminated soil
Ingestion of soil by grazing sheep has been measured in two soil types, at two stocking rates, and over two grazing seasons. Animals ingested up to 400 g soil per kg of body weight between May and November. Rainfall and stocking rate emerged as factors influencing ingestion. The effect of soil type and vegetation type was less evident.[20]
The average weight of an adult sheep is around 250 pounds.[21] If an adult sheep ate 400g/kg of soil as predicted by D. McGrath et al., then the average sheep would ingest about 45,000 g over six months, or 251 g per day. Assuming the soil was contaminated with prions (PrPSC) from feces or birth fluids, then potentially the sheep would become infected. The concentration of the prions in the soil is uncertain, and concentration is not directly proportional to infectivity. Factors affecting prion infectivity in the soil have been shown to include the length of time in the soil and the binding abilities of the soil.
For a detailed risk assessment of scrapie-contaminated soil, it was of major importance to analyze whether the detectable PrPSc in the soil extracts still exhibited oral infectivity after incubation times up to 29 months. A bioassay with Syrian hamsters was performed by feeding the animals with contaminated soil or aqueous soil extracts that had been collected after soil incubation for 26 and 29 months, respectively. Hamsters fed with contaminated soil exhibited their first scrapie-associated symptoms at two weeks to six months (95% CI) after the first feeding. The hamsters reached the terminal stage of scrapie at five to 21 months (95% CI) after the first feeding. This indicated substantial amounts of persistent infectivity in soil that had been incubated for 26 and 29 months.[17] In Iceland in 1978, a program was implemented to eradicate scrapie, and affected flocks were culled, premises were disinfected, and sheep houses were burnt; after two to three years, the premises were restocked with lambs from scrapie-free areas. Between 1978 and 2004, scrapie recurred on 33 farms. Nine recurrences occurred 14–21 years after culling as a result of persistent environmental contamination with PrPSc.[22]
The binding abilities of different soil types have been shown to enhance disease penetrance into a population. Soil containing the common clay mineral montmorillonite (Mte) and kaolinite (Kte) binds more effectively with the prions than soil containing quartz.[23] Enhanced transmissibility of soil-bound prions may explain the environmental spread of scrapie despite low levels shed into the environment. The mechanism by which Mte or other soil components enhances the transmissibility of particle bound prions remains to be clarified. Prion binding to Mte or other soil components may partially protect PrPSC from denaturation or proteolysis in the digestive tract, allowing more disease agent to be taken up from the gut. Adsorption of PrPSc soil may alter the aggregation state of the protein, shifting the size distribution toward more infectious prion protein particles, thereby increasing the infectious units. For prion disease to be transmitted via ingestion of prion contaminated soil, prions must also remain infectious by the oral route of exposure. Researchers at the University of Wisconsin investigated the oral infectivity of Mte-bound and soil-bound prions. The effects of prion source (via infected brain homogenate and purified PrPSc) and dose on penetrance (proportion of animals eventually exhibiting clinical signs of scrapie) and incubation period (time to onset of clinical symptoms) was evaluated. About 38% of animals receiving orally 200 ng of unbound, clarified PrPSc derived from soil exhibited clinical symptoms, with an incubation period for infected animals of 203 to 633 days. All animals orally dosed with an equivalent amount of Mte-bound PrPSc manifested disease symptoms in 195 to 637 days. By contrast, animals orally receiving Mte soil alone or one-tenth as much unbound clarified PrPSc (20 ng) remained asymptomatic throughout the course of the experiment. These data established that Mte-bound prions remain infectious via the oral route of exposure, and that the binding agent Mte increases disease penetrance, enhancing the efficiency of oral transmission.[24]
Transmission summary
Prions (PrPSc) are shed from sheep and goats in birth fluids, feces and other excrement. The concentration of the prions is uncertain, but is not directly proportional to infectivity. Sheep ingest a considerable amount of soil, so soil represents a plausible environmental reservoir of scrapie prions, which can persist in the environment for years. Longevity of the prions and the attachment of soil particles likely influences the persistence and infectivity of prions in the environment.
Effective methods of inactivating prions in the soil are currently lacking, and the effects of natural degradation mechanisms on prion infectivity are largely unknown. An improved understanding of the processes affecting the mobility, persistence and bioavailability of prions in soil is needed for the management of prion-contaminated environments. A system for estimating the prion-binding capacity of soil on farms using simple soil analysis may allow an estimate of the prion risk in the environment, and whether altering prion binding by the use of soil amendments may help to mitigate the infectious prions. Lichens, specifically, Parmelia sulcata, Cladonia rangiferina and Lobaria pulmonaria, may have potential for reducing the number of prions because some lichen species contain proteases that show promise in breaking down the prion. Further work to clone and characterize the proteases, assess their effects on prion infectivity, and determine which component organism or organisms present in lichens produce or influence the protease activity is warranted and is currently under investigation.[25]
See also
- Mad cow disease
- Creutzfeldt–Jakob disease
- Kuru
- Transmissible mink encephalopathy
- Virino
- Rams (film), a 2016 Icelandic drama
References
- ↑ Detwiler LA (1992). "Scrapie". Rev. - Off. Int. Epizoot. 11 (2): 491–537. PMID 1617202.
- ↑ Hunter N (2007). "Scrapie: uncertainties, biology and molecular approaches". Biochim. Biophys. Acta. 1772 (6): 619–28. doi:10.1016/j.bbadis.2007.04.007. PMID 17560089.
- ↑ National Scrapie Education Initiative. "Scrapie Fact Sheet". National Institute for Animal Agriculture. Retrieved 4 December 2011.
- ↑ Rolf, George. "From Sheep to Humans: Scrapie and Creutzfeldt–Jakob Disease". Ecclectica. Retrieved 4 December 2011.
- ↑ Foster JD, Parnham D, Chong A, Goldmann W, Hunter N (2001). "Clinical signs, histopathology and genetics of experimental transmission of BSE and natural scrapie to sheep and goats". Vet. Rec. 148 (6): 165–71. doi:10.1136/vr.148.6.165. PMID 11258721.
- ↑ Detwiler LA, Baylis M (2003). "The epidemiology of scrapie" (PDF). Rev. - Off. Int. Epizoot. 22 (1): 121–43. PMID 12793776.
- 1 2 3 4 5 Scrapie reviewed and published by WikiVet, accessed 12 October 2011.
- ↑ Tarmen viktig for skrapesyke - forskning.no
- ↑ Konold Moore; Bellworthy Simmons (2008). "Evidence of scrapie transmission via milk". BMC Veterinary Research. 4: 16. doi:10.1186/1746-6148-4-16.
- ↑ O'Rourke KI, Duncan JV, Logan JR, et al. (2002). "Active surveillance for scrapie by third eyelid biopsy and genetic susceptibility testing of flocks of sheep in Wyoming". Clin. Diagn. Lab. Immunol. 9 (5): 966–71. doi:10.1128/CDLI.9.5.966-971.2002. PMC 120069
 . PMID 12204945.
. PMID 12204945. - ↑ Eddie Straiton, "Sheep Ailments - recognition and treatment", 7th edition (2001) ISBN 1-86126-397-X
- ↑ Synnøve Vatn, Lisbeth Hektoen, Ola Nafstad "Helse og Velferd hos sau" 1. utgave, Tun Forlag (2008) ISBN 978-82-529-3180-8
- ↑ Heim D, Kihm U (2003). "Risk management of transmissible spongiform encephalopathies in Europe". Rev. - Off. Int. Epizoot. 22 (1): 179–99. PMID 12793779.
- ↑ "Detecting Prions in Blood" (PDF). Microbiology Today.: 195. August 2010. Retrieved 2011-08-21.
- ↑ "SOFIA: An Assay Platform for Ultrasensitive Detection of PrPSc in Brain and Blood" (PDF). SUNY Downstate Medical Center. Retrieved 2011-08-19.
- ↑ Saunders, Samuel E.; Shannon L. Bartelt-Hunt; Jason C. Bartz (Oct–Nov 2008/Dec). "Prions in the environment". Prion. 2 (4): 162–169. doi:10.4161/pri.2.4.7951. Check date values in:
|date=(help) - 1 2 Seidel, Bjoern; Thomzig A; Buschmann A; Groschup M; Peters R; Beekes M; Terytze K (9 May 2007). "Scrapie Agent (Strain 263K) Can Transmit Disease via the Oral Route after Persistence in Soil Over Years". PLOS ONE. 2 (5): e435. doi:10.1371/journal.pone.0000435.
- ↑ O'Rourke, Catherine. "PP - USDA ARS".
- ↑ Terry, Linda; et al. (18 May 2011). "Detection of Prions in the faeces of sheep naturally infected with classical scrapie". Veterinary Research. 42 (65).
- ↑ McGrath, D; et al. (1982). "Soil Ingestion by Grazing Sheep". Irish Journal of Agriculture.
- ↑ USDA, National Statistical Service. "Livestock Slaughter 2010".
- ↑ Georgsson, Gudmundu; et al. (2006). "Infectious agent of sheep scrapie may persist in the environment for at least 16 years". Journal of General Virology. 87: 3737–3740. doi:10.1099/vir.0.82011-0.
- ↑ O'Rourke, Katherine. "USDA-ARS 2011".
- ↑ Pederson, Joel; et al. (July 2007). "Oral transmissibility of prion disease is enhanced by binding to soil particles". PLOS Pathog.
- ↑ Johnson, CJ; et al. (2011). "Degradation of the disease-associated prion protein by a serine protease from lichens". PLOS ONE. 6: e19836. doi:10.1371/journal.pone.0019836. PMC 3092769
 . PMID 21589935.
. PMID 21589935.
External links
| Wikimedia Commons has media related to Scrapie. |
- Article about scrapie and the aforementioned diagnostic test
- UK government scrapie information
- UK government National Scrapie Plan
- Scrapie research at the Institute for Animal Health (UK)
- Sheep genetics research at the Institute for Animal Health (includes photo of a sheep with scrapie)
- Scrapie in the United States
- US Department of Agriculture video of infected sheep demonstrating Hopping Gait
- Striking a Nerve: Prions Not the Last Word in TSEs – opinion article by Frank Bastian that proposes a different causation for scrapie and other prion diseases