Silver thiocyanate
| | |
 | |
| Names | |
|---|---|
| IUPAC name
Silver(I) thiocyanate, Silver thiocyanate | |
| Other names
Thiocyanic acid, silver (1+) thiocyanate; Silver isothiocyanate; Silver sulphocyanide[1] | |
| Identifiers | |
| 1701-93-5 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 66941 |
| ECHA InfoCard | 100.015.395 |
| EC Number | 216-934-9 |
| PubChem | 74345 |
| UNII | S44O8TME5U |
| UN number | 3077 |
| |
| |
| Properties | |
| CAgNS | |
| Molar mass | 165.95 g·mol−1 |
| Appearance | Colorless crystals |
| Odor | Odorless |
| Melting point | 170 °C (338 °F; 443 K) decomposes[2] |
| 0.14 mg/L (19.96 °C) 0.25 mg/L (21 °C) 6.68 mg/L (100 °C)[1] | |
| Solubility product (Ksp) |
1.03·10−12[3] |
| Solubility | Insoluble in acids (reacts)[4] except when concentrated, acetates, aq. nitrates[1] |
| Solubility in silver nitrate | 43.2 mg/L (25.2 °C, 3 nAgNO3/H2O)[1] |
| Solubility in sulfur dioxide | 14 mg/kg (0 °C)[2] |
| Solubility in methanol | 0.0022 mg/kg[2] |
| −6.18·10−5 cm3/mol[3] | |
| Structure | |
| Monoclinic, mS32 (293 K)[5] | |
| C2/c, No. 15 (293 K)[5] | |
| 2/m (293 K)[5] | |
| α = 90°, β = 93.75(1)°, γ = 90° | |
| Thermochemistry | |
| 63 J/mol·K[2] | |
| Std molar entropy (S |
131 J/mol·K[2] |
| Std enthalpy of formation (ΔfH |
88 kJ/mol[2] |
| Hazards | |
| GHS pictograms |   [4] [4] |
| GHS signal word | Warning |
| H302, H312, H332, H410[4] | |
| P273, P280, P501[4] | |
| EU classification (DSD) |
|
| R-phrases | R20/21/22, R32, R50/53 |
| S-phrases | S13, S36/37, S46, S60, S61 |
| NFPA 704 | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Silver thiocyanate is a silver salt of thiocyanic acid with the formula AgSCN.
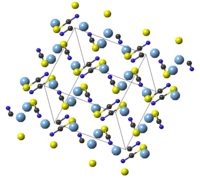
Structure
AgSCN is monoclinic with 8 molecules in the cell. The SCN− groups has an almost linear molecular geometry, with bond angle 179.6(5)°. Weak Ag—Ag interactions length are 0.3249(2) nm to 0.3338(2) nm which forming one-dimensional zig-zag chain in AgSCN.[5]
Production
Silver thiocyanate is produced by the reaction between silver nitrate and potassium thiocyanate.
References
- 1 2 3 4 Comey, Arthur Messinger; Hahn, Dorothy A. (1921-02). A Dictionary of Chemical Solubilities: Inorganic (2nd ed.). New York: The MacMillan Company. p. 884. Check date values in:
|date=(help) - 1 2 3 4 5 6 Anatolievich, Kiper Ruslan. "silver thiocyanate". http://chemister.ru. Retrieved 2014-07-19. External link in
|website=(help) - 1 2 Lide, David R., ed. (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- 1 2 3 4 Sigma-Aldrich Co., Silver thiocyanate. Retrieved on 2014-07-19.
- 1 2 3 4 5 Zhu, H.-L.; Liu, G.-F.; Meng, F.-J. (2003). "Refinement of the crystal structure of silver(I) thiocyanate, AgSCN". Zeitschrift für Kristallographie - New Crystal Structures. München: Oldenbourg Wissenschaftsverlag GmbH. 218 (JG): 263–264. doi:10.1524/ncrs.2003.218.jg.285. ISSN 2197-4578.
This article is issued from Wikipedia - version of the 11/2/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.
