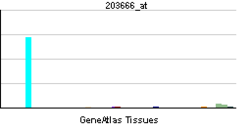
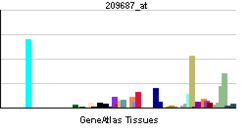
Stromal cell-derived factor 1
| View/Edit Human | View/Edit Mouse |
The stromal cell-derived factor 1 (SDF1), also known as C-X-C motif chemokine 12 (CXCL12), is a chemokine protein that in humans is encoded by the CXCL12 gene on chromosome 10.[3] It is ubiquitously expressed in many tissues and cell types.[4] Stromal cell-derived factors 1-alpha and 1-beta are small cytokines that belong to the chemokine family, members of which activate leukocytes and are often induced by proinflammatory stimuli such as lipopolysaccharide, TNF, or IL1. The chemokines are characterized by the presence of 4 conserved cysteines that form 2 disulfide bonds. They can be classified into 2 subfamilies. In the CC subfamily, the cysteine residues are adjacent to each other. In the CXC subfamily, they are separated by an intervening amino acid. The SDF1 proteins belong to the latter group.[3] CXCL12 signaling has been observed in several cancers.[5][6] The CXCL12 gene also contains one of 27 SNPs associated with increased risk of coronary artery disease.[7]
Structure
Gene
The CXCL12 gene resides on chromosome 10 at the band 10q11.1 and contains 9 exons.[3] This gene produces 7 isoforms through alternative splicing.[8]
Protein
This protein belongs to the intercrine alpha (chemokine CXC) family.[8] SDF-1 is produced in two forms, SDF-1α/CXCL12a and SDF-1β/CXCL12b, by alternate splicing of the same gene.[9] Chemokines are characterized by the presence of four conserved cysteines, which form two disulfide bonds. The CXCL12 proteins belong to the group of CXC chemokines, whose initial pair of cysteines are separated by one intervening amino acid. In addition, the first 8 residues of the CXCL12 N-terminal serve as a receptor binding site, though only Lys-1 and Pro-2 directly participated in activating the receptor. Meanwhile, the RFFESH motif (residues 12-17) in the loop region function as a docking site for CXCL12 receptor binding.[10]
Function
CXCL12 is expressed in many tissues in mice including brain, thymus, heart, lung, liver, kidney, spleen, and bone marrow.[11] CXCL12 is strongly chemotactic for lymphocytes.[12][13][14][15] During embryogenesis, it directs the migration of hematopoietic cells from fetal liver to bone marrow and the formation of large blood vessels. It has also been shown that CXCL12 signalling regulates the expression of CD20 on B cells.[16] CXCL12 is also chemotactic for mesenchymal stem cells and is expressed in the area of inflammatory bone destruction, where it mediates their suppressive effect on osteoclastogenesis.[17]
In adulthood, CXCL12 plays an important role in angiogenesis by recruiting endothelial progenitor cells (EPCs) from the bone marrow through a CXCR4 dependent mechanism.[18]
CXCR4, previously called LESTR or fusin, is the receptor for CXCL12.[12] This CXCL12-CXCR4 interaction used to be considered exclusive (unlike for other chemokines and their receptors), but recently, it was suggested that CXCL12 may also bind the CXCR7 receptor.[19][20][21] By blocking CXCR4, a major coreceptor for HIV-1 entry, CXCL12 acts as an endogenous inhibitor of CXCR4-tropic HIV-1 strains.[22]
Clinical significance
In humans, CXCL12 has been implicated in a wide variety of biomedical conditions involving several organ systems.[23] Chemokines and chemokine receptors, of which CXCR stands out, regulate multiple processes such as morphogenesis, angiogenesis, and immune responses and are considered potential targets for drug development. In the gastrointestinal tract system, the CXCL12-CXCR4 axis is under investigation as an anti-fibrotic therapy in the treatment for chronic pancreatitis.[24] For instance, blocking CXCR4, the receptor for CXCL12, with Plerixafor (AMD-3100) increased the effectiveness of combretastatin in a mouse model of breast cancer, presumably by preventing macrophages from being recruited to tumours.[15][16] Furthermore, CXCL12 signaling in conjunction with CXCR7 signaling has been implicated in the progression of pancreatic cancer.[5] In the urinary tract system, methylation of the CXCL12 promoter and expression of PD-L1 may be powerful prognostic biomarkers for biochemical recurrence in prostate carcinoma patients after radical prostatectomy, and further studies are ongoing to confirm if CXCL12 methylation may aid in active surveillance strategies.[25] In the field of oncology, melanoma associated fibroblasts are stimulated by stimulation of the A2B adenosine receptor followed by stimulation of fibroblast growth factor and increased expression of CXCL12.[6]
Clinical marker
A multi-locus genetic risk score study based on a combination of 27 loci, including the CXCL12 gene, identified individuals at increased risk for both incident and recurrent coronary artery disease events, as well as an enhanced clinical benefit from statin therapy. The study was based on a community cohort study (the Malmo Diet and Cancer study) and four additional randomized controlled trials of primary prevention cohorts (JUPITER and ASCOT) and secondary prevention cohorts (CARE and PROVE IT-TIMI 22).[7]
References
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- 1 2 3 "Entrez Gene: CXCL12 chemokine (C-X-C motif) ligand 12 (stromal cell-derived factor 1)".
- ↑ "BioGPS - your Gene Portal System". biogps.org. Retrieved 2016-10-11.
- 1 2 Guo JC, Li J, Zhou L, Yang JY, Zhang ZG, Liang ZY, Zhou WX, You L, Zhang TP, Zhao YP (August 2016). "CXCL12-CXCR7 axis contributes to the invasive phenotype of pancreatic cancer". Oncotarget. doi:10.18632/oncotarget.11330. PMID 27542220.
- 1 2 Sorrentino C, Miele L, Porta A, Pinto A, Morello S (August 2016). "Activation of the A2B adenosine receptor in B16 melanomas induces CXCL12 expression in FAP-positive tumor stromal cells, enhancing tumor progression". Oncotarget. doi:10.18632/oncotarget.11729. PMID 27590504.
- 1 2 Mega JL, Stitziel NO, Smith JG, Chasman DI, Caulfield MJ, Devlin JJ, Nordio F, Hyde CL, Cannon CP, Sacks FM, Poulter NR, Sever PS, Ridker PM, Braunwald E, Melander O, Kathiresan S, Sabatine MS (June 2015). "Genetic risk, coronary heart disease events, and the clinical benefit of statin therapy: an analysis of primary and secondary prevention trials". Lancet. 385 (9984): 2264–71. doi:10.1016/S0140-6736(14)61730-X. PMID 25748612.
- 1 2 "CXCL12 - Stromal cell-derived factor 1 precursor - Homo sapiens (Human) - CXCL12 gene & protein". UniProt.
- ↑ De La Luz Sierra M, Yang F, Narazaki M, Salvucci O, Davis D, Yarchoan R, Zhang HH, Fales H, Tosato G (April 2004). "Differential processing of stromal-derived factor-1alpha and stromal-derived factor-1beta explains functional diversity". Blood. 103 (7): 2452–9. doi:10.1182/blood-2003-08-2857. PMID 14525775.
- ↑ Crump MP, Gong JH, Loetscher P, Rajarathnam K, Amara A, Arenzana-Seisdedos F, Virelizier JL, Baggiolini M, Sykes BD, Clark-Lewis I (December 1997). "Solution structure and basis for functional activity of stromal cell-derived factor-1; dissociation of CXCR4 activation from binding and inhibition of HIV-1". The EMBO Journal. 16 (23): 6996–7007. doi:10.1093/emboj/16.23.6996. PMID 9384579.
- ↑ Schrader AJ, Lechner O, Templin M, Dittmar KE, Machtens S, Mengel M, Probst-Kepper M, Franzke A, Wollensak T, Gatzlaff P, Atzpodien J, Buer J, Lauber J (April 2002). "CXCR4/CXCL12 expression and signalling in kidney cancer". British Journal of Cancer. 86 (8): 1250–6. doi:10.1038/sj.bjc.6600221. PMC 2375348
 . PMID 11953881.
. PMID 11953881. - 1 2 Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA (September 1996). "A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1)". The Journal of Experimental Medicine. 184 (3): 1101–9. doi:10.1084/jem.184.3.1101. PMC 2192798
 . PMID 9064327.
. PMID 9064327. - ↑ Ara T, Nakamura Y, Egawa T, Sugiyama T, Abe K, Kishimoto T, Matsui Y, Nagasawa T (April 2003). "Impaired colonization of the gonads by primordial germ cells in mice lacking a chemokine, stromal cell-derived factor-1 (SDF-1)". Proceedings of the National Academy of Sciences of the United States of America. 100 (9): 5319–23. doi:10.1073/pnas.0730719100. PMC 154343
 . PMID 12684531.
. PMID 12684531. - ↑ Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS (August 2003). "Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy". Lancet. 362 (9385): 697–703. doi:10.1016/S0140-6736(03)14232-8. PMID 12957092.
- ↑ Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA (August 1998). "Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice". Proceedings of the National Academy of Sciences of the United States of America. 95 (16): 9448–53. doi:10.1073/pnas.95.16.9448. PMC 21358
 . PMID 9689100.
. PMID 9689100. - ↑ Pavlasova G, Borsky M, Seda V, Cerna K, Osickova J, Doubek M, Mayer J, Calogero R, Trbusek M, Pospisilova S, Davids MS, Kipps TJ, Brown JR, Mraz M (August 2016). "Ibrutinib inhibits CD20 up-regulation on CLL B cells mediated by the CXCR4/SDF-1 axis". Blood. doi:10.1182/blood-2016-04-709519. PMID 27480113.
- ↑ Takano T, Li YJ, Kukita A, Yamaza T, Ayukawa Y, Moriyama K, Uehara N, Nomiyama H, Koyano K, Kukita T (2014). "Mesenchymal stem cells markedly suppress inflammatory bone destruction in rats with adjuvant-induced arthritis". Laboratory Investigation; a Journal of Technical Methods and Pathology. 94 (3): 286–96. doi:10.1038/labinvest.2013.152. PMID 24395111.
- ↑ Zheng H, Fu G, Dai T, Huang H (2007). "Migration of endothelial progenitor cells mediated by stromal cell-derived factor-1alpha/CXCR4 via PI3K/Akt/eNOS signal transduction pathway". Journal of Cardiovascular Pharmacology. 50 (3): 274–80. doi:10.1097/FJC.0b013e318093ec8f. PMID 17878755.
- ↑ Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, Arenzana-Seisdedos F, Thelen M, Bachelerie F (2005). "The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes". The Journal of Biological Chemistry. 280 (42): 35760–6. doi:10.1074/jbc.M508234200. PMID 16107333.
- ↑ Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ, Wei K, McMaster BE, Wright K, Howard MC, Schall TJ (2006). "A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development". The Journal of Experimental Medicine. 203 (9): 2201–13. doi:10.1084/jem.20052144. PMC 2118398
 . PMID 16940167.
. PMID 16940167. - ↑ Cruz-Orengo L, Holman DW, Dorsey D, Zhou L, Zhang P, Wright M, McCandless EE, Patel JR, Luker GD, Littman DR, Russell JH, Klein RS (2011). "CXCR7 influences leukocyte entry into the CNS parenchyma by controlling abluminal CXCL12 abundance during autoimmunity". The Journal of Experimental Medicine. 208 (2): 327–39. doi:10.1084/jem.20102010. PMC 3039853
 . PMID 21300915.
. PMID 21300915. - ↑ Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier JL, Arenzana-Seisdedos F, Schwartz O, Heard JM, Clark-Lewis I, Legler DF, Loetscher M, Baggiolini M, Moser B (1996). "The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1". Nature. 382 (6594): 833–5. doi:10.1038/382833a0. PMID 8752281.
- ↑ Pozzobon T, Goldoni G, Viola A, Molon B (September 2016). "CXCR4 signaling in health and disease". Immunology Letters. 177: 6–15. doi:10.1016/j.imlet.2016.06.006. PMID 27363619.
- ↑ Neesse A, Ellenrieder V (September 2016). "NEMO-CXCL12/CXCR4 axis: a novel vantage point for antifibrotic therapies in chronic pancreatitis?". Gut. doi:10.1136/gutjnl-2016-312874. PMID 27590996.
- ↑ Goltz D, Holmes EE, Gevensleben H, Sailer V, Dietrich J, Jung M, Röhler M, Meller S, Ellinger J, Kristiansen G, Dietrich D (July 2016). "CXCL12 promoter methylation and PD-L1 expression as prognostic biomarkers in prostate cancer patients". Oncotarget. doi:10.18632/oncotarget.10786. PMID 27462860.
Further reading
- Kucia M, Reca R, Miekus K, Wanzeck J, Wojakowski W, Janowska-Wieczorek A, Ratajczak J, Ratajczak MZ (August 2005). "Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis". Stem Cells. 23 (7): 879–94. doi:10.1634/stemcells.2004-0342. PMID 15888687.
- Kryczek I, Wei S, Keller E, Liu R, Zou W (March 2007). "Stroma-derived factor (SDF-1/CXCL12) and human tumor pathogenesis". American Journal of Physiology. Cell Physiology. 292 (3): C987–95. doi:10.1152/ajpcell.00406.2006. PMID 16943240.
- Stellos K, Gawaz M (March 2007). "Platelets and stromal cell-derived factor-1 in progenitor cell recruitment". Seminars in Thrombosis and Hemostasis. 33 (2): 159–64. doi:10.1055/s-2007-969029. PMID 17340464.
- Wang J, Liu X, Lu H, Jiang C, Cui X, Yu L, Fu X, Li Q, Wang J (March 2015). "CXCR4(+)CD45(-) BMMNC subpopulation is superior to unfractionated BMMNCs for protection after ischemic stroke in mice". Brain, Behavior, and Immunity. 45: 98–108. doi:10.1016/j.bbi.2014.12.015. PMC 4342301
 . PMID 25526817.
. PMID 25526817. - Arya M, Ahmed H, Silhi N, Williamson M, Patel HR (2007). "Clinical importance and therapeutic implications of the pivotal CXCL12-CXCR4 (chemokine ligand-receptor) interaction in cancer cell migration". Tumour Biology. 28 (3): 123–31. doi:10.1159/000102979. PMID 17510563.