Teichoic acid
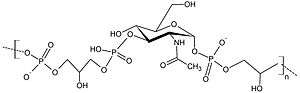
Teichoic acids (cf. Greek τεῖχος, teīkhos, "wall", to be specific a fortification wall, as opposed to τοῖχος, toīkhos, a regular wall)[1] are bacterial copolymers [2] of glycerol phosphate or ribitol phosphate and carbohydrates linked via phosphodiester bonds.
Location and structure
Teichoic acids are found within the cell wall of most Gram-positive bacteria such as species in the genera Staphylococcus, Streptococcus, Bacillus, Clostridium, Corynebacterium, and Listeria, and appear to extend to the surface of the peptidoglycan layer. Teichoic acids are not found in Gram-negative bacteria. They can be covalently linked to N-acetylmuramic acid or a terminal D-alanine in the tetrapeptide crosslinkage between N-acetylmuramic acid units of the peptidoglycan layer, or they can be anchored in the cytoplasmic membrane with a lipid anchor.
Teichoic acids that are anchored to the lipid membrane are referred to as lipoteichoic acids (LTAs), whereas teichoic acids that are covalently bound to peptidoglycan are referred to as wall teichoic acids (WTA).[3]
Wall teichoic acids
The most common structures are a ManNAc(β1→4)GlcNAc disaccharide with one to three glycerol phosphates attached to the C4 hydroxyl of the ManNAc residue followed by a long chain of glycerol- or ribitol phosphate repeats.[3]
Function
The main function of teichoic acids is to provide rigidity to the cell-wall by attracting cations such as magnesium and sodium. Teichoic acids can be substituted with D-alanine ester residues,[4] or D-glucosamine,[5] giving the molecule zwitterionic properties.[6] These zwitterionic teichoic acids are suspected ligands for toll-like receptors 2 and 4. Teichoic acids also assist in regulation of cell growth by limiting the ability of autolysins to break the β(1-4) bond between the N-acetyl glucosamine and the N-acetylmuramic acid.
Lipoteichoic acids may also act as receptor molecules for some Gram-positive bacteriophage; however, this has not yet been conclusively supported.[7]
Biosynthesis
Enzymes involved in the biosynthesis of WTAs have been named : TarO, TarA, TarB, TarF, TarK, and TarL.[3]
Wall Teichoic Acids synthesis as an antibiotic drug target
This was proposed in 2004.[3]
See also
- Lipoteichoic acid - a major constituent of the cell wall of gram-positive bacteria
- Sir James Baddiley
References
- ↑ τεῖχος. Liddell, Henry George; Scott, Robert; A Greek–English Lexicon at the Perseus Project
- ↑ Teichoic acids at the US National Library of Medicine Medical Subject Headings (MeSH)
- 1 2 3 4 Wall Teichoic Acid Function, Biosynthesis, and Inhibition. Jan 2010
- ↑ Knox KW, Wicken AJ (June 1973). "Immunological properties of teichoic acids". Bacteriological Reviews. 37 (2): 215–57. PMC 413812
 . PMID 4578758.
. PMID 4578758. - ↑ Cot M, Nigou J, et al. (October 2011). "Lipoteichoic Acid in Streptomyces hygroscopicus:Structural Model and Immunomodulatory Activities". PLOS ONE. 6 (10). doi:10.1371/journal.pone.0026316.
- ↑ Garimella R, Halye JL, Harrison W, Klebba PE, Rice CV (October 2009). "Conformation of the phosphate D-alanine zwitterion in bacterial teichoic acid from nuclear magnetic resonance spectroscopy". Biochemistry. 48 (39): 9242–9. doi:10.1021/bi900503k. PMID 19746945.
- ↑ Raisanen, L.; Draing, C.; Pfizenmaier, M.; Schubert, K.; Jaakonsaari, T.; Aulouck, S.; Hartung, T. & Alatossava, T. (June 2007). "Molecular interaction between lipoteichoic acids and Lactobacillus delbrueckii phages depends on the D-Alanyl and a-glucose substitution of poly(glycerophosphate) backbones". J.Bac: 4135–4140.