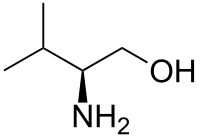
Valinol
 | |
| Names | |
|---|---|
| IUPAC name
(S)-(+)-2-Amino-3-methyl-1-butanol | |
| Other names
(2S)-2-Amino-3-methyl-butan-1-ol | |
| Identifiers | |
| 16369-05-4 (DL) 2026-48-4 (L) 4276-09-9 (D) | |
| 3D model (Jmol) | (DL): Interactive image (L): Interactive image (D): Interactive image |
| ChemSpider | 71352 (DL) 556322 (L) 5323522 (D) |
| ECHA InfoCard | 100.016.342 |
| PubChem | 79019 (DL) 640993 (L) 6950587 (D) |
| |
| |
| Properties | |
| C5H13NO | |
| Molar mass | 103.17 g·mol−1 |
| Appearance | White to yellow crystalline powder |
| Density | 0.926 g/mL |
| Melting point | 30 to 34 °C (86 to 93 °F; 303 to 307 K) |
| Boiling point | 189 to 190 °C (372 to 374 °F; 462 to 463 K) |
| Very soluble | |
| Hazards | |
| Safety data sheet | Sigma Aldrich[1] |
| GHS pictograms |  |
| GHS signal word | Warning |
| H315, H319, H335[1] | |
| P261, P305+351+338[1] | |
| Flash point | 90°C [1] closed cup |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Valinol is an organic compound named after, and commonly produced from, the amino acid valine. The compound is chiral and is produced almost exclusively as the S‑isomer (also designated as the L‑isomer), due to the abundant supply of S-valine. It is part of a broader class of amino alcohols.
Synthesis
Valinol can be generated by converting the carboxylic group of valine to an alcohol with a strong reducing agent such as lithium aluminium hydride,[2] or with NaBH4 and I2 (forming the borane–tetrahydrofuran complex).[3] In both cases the valinol produced can be subsequently purified by short path distillation.
Reactions
Valinol is mainly used to prepare chiral oxazolines, a process which can be achieved via a variety of methods. These oxazolines are principally used as ligands in asymmetric catalysis.[4]
See also
- (S)-iPr-PHOX - an oxazoline ligand made using valinol
References
- 1 2 3 4 Sigma-Aldrich Co., 2-Amino-3-methyl-1-butanol. Retrieved on 2014-10-22.
- ↑ Dickman, D.A.; Meyers, A.I.; Smith, G.A.; Gawley, R.E. (1990). "Reduction of α-Amino Acids". Organic Syntheses. 7: 530. Retrieved 11 October 2012.
- ↑ McKennon, Marc J.; Meyers, A. I.; Drauz, Karlheinz; Schwarm, Michael (1993). "A convenient reduction of amino acids and their derivatives". The Journal of Organic Chemistry. 58 (13): 3568–3571. doi:10.1021/jo00065a020.
- ↑ McManus, Helen A.; Guiry, Patrick J. (Sep 2004). "Recent Developments in the Application of Oxazoline-Containing Ligands in Asymmetric Catalysis". Chemical Reviews. 104 (9): 4151–4202. doi:10.1021/cr040642v. PMID 15352789.