Alpha-enolase
| View/Edit Human | View/Edit Mouse |
Enolase 1 (ENO1), more commonly known as alpha-enolase, is a glycolytic enzyme expressed in most tissues, one of the isozymes of enolase. Each isoenzyme is a homodimer composed of 2 alpha, 2 gamma, or 2 beta subunits, and functions as a glycolytic enzyme. Alpha-enolase, in addition, functions as a structural lens protein (tau-crystallin) in the monomeric form. Alternative splicing of this gene results in a shorter isoform that has been shown to bind to the c-myc promoter and function as a tumor suppressor. Several pseudogenes have been identified, including one on the long arm of chromosome 1. Alpha-enolase has also been identified as an autoantigen in Hashimoto encephalopathy.[3]
Structure
ENO1 is one of three enolase isoforms, the other two being ENO2 (ENO-γ) and ENO3 (ENO-β).[4] Each isoform is a protein subunit that can hetero- or homodimerize to form αα, αβ, αγ, ββ, and γγ dimers.[5] The ENO1 gene spans 18 kb and lacks a TATA box while possessing multiple transcription start sites.[6] A hypoxia-responsive element can be found in the ENO1 promoter and allows the enzyme to function in aerobic glycolysis and contribute to the Warburg effect in tumor cells.[7]
Relationship to Myc-binding protein-1
The mRNA transcript of the ENO1 gene can be alternatively translated into a cytoplasmic protein, with a molecular weight of 48 kDa, or a nuclear protein, with a molecular weight of a 37 kDa.[7][8] The nuclear form was previously identified as Myc-binding protein-1 (MBP1), which downregulates the protein level of the c-myc protooncogene.[8][9] A start codon at codon 97 of ENO1 and a Kozak consensus sequence were found preceding the 3' region of ENO1 encoding the MBP1 protein. In addition, the N-terminal region of the MBP1 protein it critical to DNA binding and, thus, its inhibitory function.[8]
Function
As an enolase, ENO1 is a glycolytic enzyme the catalyzes the conversion of 2-phosphoglycerate to phosphoenolpyruvate.[4][7][10] This isozyme is ubiquitously expressed in adult human tissues, including liver, brain, kidney, and spleen.[4] Within cells, ENO1 predominantly localizes to the cytoplasm, though an alternatively translated form is localizes to the nucleus.[4][7] Its nuclear form, also known as MBP1, functions solely as a tumor suppressor by binding and inhibiting the c-myc protooncogene promoter, and lacks the glycolytic enzyme activity of the cytoplasmic form.[8] ENO1 also plays a role in other functions, including a cell surface receptor for plasminogen on pathogens, such as streptococci, and activated immune cells, leading to systemic infection or tissue invasion; an oxidative stress protein in endothelial cells; a lens crystalline; a heat shock protein; and a binding partner of cytoskeletal and chromatin structures to aid in transcription.[7][8][10][11][12]
Clinical significance
Cancer
ENO1 overexpression has been associated with multiple tumors, including glioma, neuroendocrine tumors, neuroblastoma, pancreatic cancer, prostate cancer, cholangiocarcinoma, thyroid carcinoma, lung cancer, hepatocellular carcinoma, and breast cancer.[4][7][12][13] In many of these tumors, ENO1 promoted cell proliferation by regulating the PI3K/AKT signaling pathway and induced tumorigenesis by activating plasminogen.[4][7] Moreover, ENO1 is expressed on the tumor cell surface during pathological conditions such as inflammation, autoimmunity, and malignancy. Its role as a plasminogen receptor leads to extracellular matrix degradation and cancer invasion.[7][12][13] Due to its surface expression, targeting surface ENO1 enables selective targeting of tumor cells while leaving the ENO1 inside normal cells functional.[7] Moreover, in tumors such as Non-Hodgkin's Lymphomas (NHLs) and breast cancer, inhibition of ENO1 expression decreased tolerance to hypoxia while increasing sensitivity to radiation therapy, thus indicating that ENO1 may have aided chemoresistance.[4][10] Considering these factors, ENO1 holds great potential to serve as an effective therapeutic target for treating many types of tumors in patients.[4][10][12]
Autoimmune disease
ENO1 has been detected in serum drawn from children diagnosed with juvenile idiopathic arthritis.[14]
Alpha-enolase has been identified as an autoantigen in Hashimoto's encephalopathy.[15] Single studies have also identified it as an autoantigen associated with severe asthma[16] and a putative target antigen of anti-endothelial cell antibody in Behçet's disease.[17] Reduced expression of the enzyme has been found in the corneal epithelium of people suffering from keratoconus.[18][19]
Gastrointestinal disease
CagA protein was found to activate ENO1 expression through activating the Src and MEK/ERK pathways as a mechanism for H. pylori-mediated gastric diseases.[13]
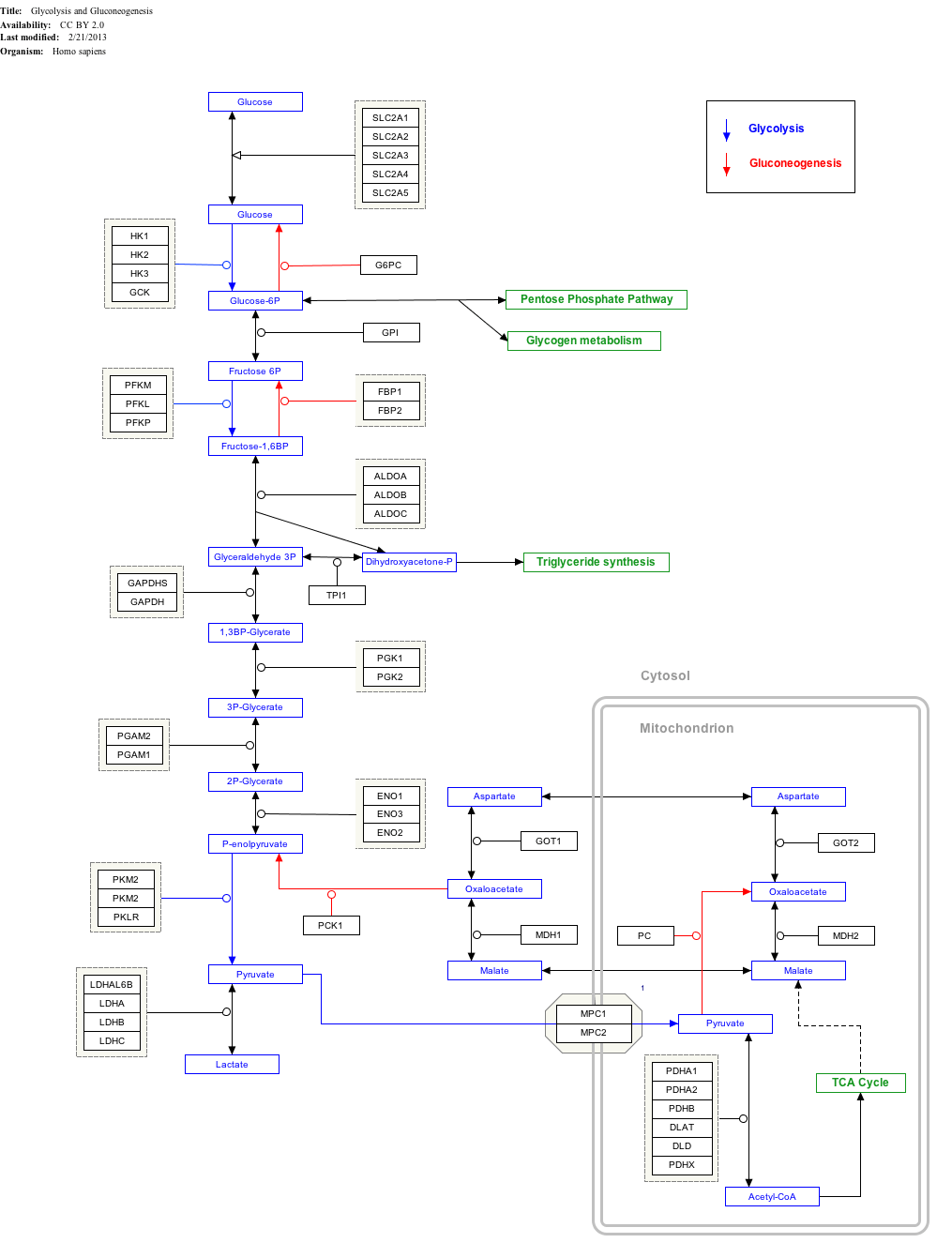
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
Glycolysis and Gluconeogenesis edit
- ↑ The interactive pathway map can be edited at WikiPathways: "GlycolysisGluconeogenesis_WP534".
Interactions
Alpha-enolase has been shown to interact with TRAPPC2.[20]
See also
External links
- Alpha-Enolase Linked to Severe Asthma - medscape news report, 25 aug 2006.
References
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ "ENO1 enolase 1 (alpha)". NCBI Entrez Gene database.
- 1 2 3 4 5 6 7 8 Zhu X, Miao X, Wu Y, Li C, Guo Y, Liu Y, Chen Y, Lu X, Wang Y, He S (Jul 2015). "ENO1 promotes tumor proliferation and cell adhesion mediated drug resistance (CAM-DR) in Non-Hodgkin's Lymphomas". Experimental Cell Research. 335 (2): 216–23. doi:10.1016/j.yexcr.2015.05.020. PMID 26024773.
- ↑ Kim AY, Lim B, Choi J, Kim J (Aug 2015). "The TFG-TEC oncoprotein induces transcriptional activation of the human β-enolase gene via chromatin modification of the promoter region". Molecular Carcinogenesis. doi:10.1002/mc.22384. PMID 26310886.
- ↑ Giallongo A, Venturella S, Oliva D, Barbieri G, Rubino P, Feo S (Jun 1993). "Structural features of the human gene for muscle-specific enolase. Differential splicing in the 5'-untranslated sequence generates two forms of mRNA". European Journal of Biochemistry / FEBS. 214 (2): 367–74. doi:10.1111/j.1432-1033.1993.tb17932.x. PMID 8513787.
- 1 2 3 4 5 6 7 8 9 Song Y, Luo Q, Long H, Hu Z, Que T, Zhang X, Li Z, Wang G, Yi L, Liu Z, Fang W, Qi S (21 March 2014). "Alpha-enolase as a potential cancer prognostic marker promotes cell growth, migration, and invasion in glioma". Molecular Cancer. 13: 65. doi:10.1186/1476-4598-13-65. PMC 3994408
 . PMID 24650096.
. PMID 24650096. - 1 2 3 4 5 Subramanian A, Miller DM (Feb 2000). "Structural analysis of alpha-enolase. Mapping the functional domains involved in down-regulation of the c-myc protooncogene". The Journal of Biological Chemistry. 275 (8): 5958–65. doi:10.1074/jbc.275.8.5958. PMID 10681589.
- ↑ Subramanian A, Miller DM (Feb 2000). "Structural analysis of alpha-enolase. Mapping the functional domains involved in down-regulation of the c-myc protooncogene". The Journal of Biological Chemistry. 275 (8): 5958–65. doi:10.1074/jbc.275.8.5958. PMID 10681589.
- 1 2 3 4 Gao J, Zhao R, Xue Y, Niu Z, Cui K, Yu F, Zhang B, Li S (Apr 2013). "Role of enolase-1 in response to hypoxia in breast cancer: exploring the mechanisms of action". Oncology Reports. 29 (4): 1322–32. doi:10.3892/or.2013.2269. PMID 23381546.
- ↑ Pancholi V (Jun 2001). "Multifunctional alpha-enolase: its role in diseases". Cellular and Molecular Life Sciences. 58 (7): 902–20. doi:10.1007/pl00000910. PMID 11497239.
- 1 2 3 4 Hsiao KC, Shih NY, Fang HL, Huang TS, Kuo CC, Chu PY, Hung YM, Chou SW, Yang YY, Chang GC, Liu KJ (2013). "Surface α-enolase promotes extracellular matrix degradation and tumor metastasis and represents a new therapeutic target". PLOS ONE. 8 (7): e69354. doi:10.1371/journal.pone.0069354. PMC 3716638
 . PMID 23894455.
. PMID 23894455. - 1 2 3 Chen S, Duan G, Zhang R, Fan Q (Aug 2014). "Helicobacter pylori cytotoxin-associated gene A protein upregulates α-enolase expression via Src/MEK/ERK pathway: implication for progression of gastric cancer". International Journal of Oncology. 45 (2): 764–70. doi:10.3892/ijo.2014.2444. PMID 24841372.
- ↑ Moore TL, Gillian BE, Crespo-Pagnussat S, Feller L, Chauhan AK (2014). "Measurement and evaluation of isotypes of anti-citrullinated fibrinogen and anti-citrullinated alpha-enolase antibodies in juvenile idiopathic arthritis". Clinical and Experimental Rheumatology. 32 (5): 740–6. PMID 25068682.
- ↑ Yoneda M, Fujii A, Ito A, Yokoyama H, Nakagawa H, Kuriyama M (2007). "High prevalence of serum autoantibodies against the amino terminal of alpha-enolase in Hashimoto's encephalopathy". J. Neuroimmunol. 185 (1-2): 195–200. doi:10.1016/j.jneuroim.2007.01.018. PMID 17335908.
- ↑ Nahm DH, Lee KH, Shin JY, Ye YM, Kang Y, Park HS (2006). "Identification of alpha-enolase as an autoantigen associated with severe asthma". J. Allergy Clin. Immunol. 118 (2): 376–81. doi:10.1016/j.jaci.2006.04.002. PMID 16890761.
- ↑ Lee KH, Chung HS, Kim HS, Oh SH, Ha MK, Baik JH, Lee S, Bang D (2003). "Human alpha-enolase from endothelial cells as a target antigen of anti-endothelial cell antibody in Behçet's disease". Arthritis Rheum. 48 (7): 2025–35. doi:10.1002/art.11074. PMID 12847697.
- ↑ Srivastava OP, Chandrasekaran D, Pfister RR (2006). "Molecular changes in selected epithelial proteins in human keratoconus corneas compared to normal corneas". Mol. Vis. 12: 1615–25. PMID 17200661.
- ↑ Nielsen K, Vorum H, Fagerholm P, Birkenkamp-Demtröder K, Honoré B, Ehlers N, Orntoft TF (2006). "Proteome profiling of corneal epithelium and identification of marker proteins for keratoconus, a pilot study". Exp. Eye Res. 82 (2): 201–9. doi:10.1016/j.exer.2005.06.009. PMID 16083875.
- ↑ Ghosh AK, Majumder M, Steele R, White RA, Ray RB (Jan 2001). "A novel 16-kilodalton cellular protein physically interacts with and antagonizes the functional activity of c-myc promoter-binding protein 1". Molecular and Cellular Biology. 21 (2): 655–62. doi:10.1128/MCB.21.2.655-662.2001. PMC 86643
 . PMID 11134351.
. PMID 11134351.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.