Amadori rearrangement
The Amadori rearrangement is an organic reaction describing the acid or base catalyzed isomerization or rearrangement reaction of the N-glycoside of an aldose or the glycosylamine to the corresponding 1-amino-1-deoxy-ketose.[1][2] The reaction is important in carbohydrate chemistry.
The reaction mechanism is demonstrated starting from the reaction of D-mannose in its closed (1) and open-form (2) with ammonia to produce the 1,1-amino-alcohol (3), which is unstable and loses water to the glycosylamine (again the open imine (5) and the closed form hemiaminal (4)), which is the starting point for the actual Amadori rearrangement.[3]
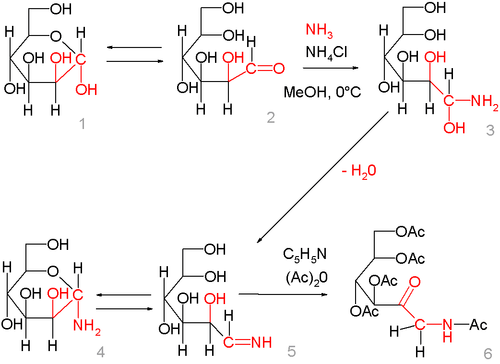
By treatment of the glycosylamine with pyridine and acetic anhydride, the imine group rearranges and the intermediate enol, in turn, rearranges to the ketone. In this particular reaction, all the alcohol and amino groups are acylated as well.
The reaction is associated with the Maillard reaction in which the reagents are naturally occurring sugars and amino acids. Recent study is revealed the possibility of Amadori rearrangement during interaction between oxidized dextran and gelatine.[4]
Amadori product
An Amadori product is an intermediate in the production of an advanced glycation end-product (AGE) as a result of glycation.
The formation of an advanced glycation end-product involves the following steps:
- Formation of a Schiff base: for example the aldehyde group of a glucose molecule will combine with the amino group of a lysine molecule (in a protein) to form an imine or Schiff base, which is a double bond between the carbon atom of the glucose and the nitrogen atom of the lysine.
- Formation of an Amadori product: the Amadori product is a re-arrangement from the Schiff base, wherein the hydrogen atom from the hydroxyl group adjacent to the carbon-nitrogen double bond moves to bond to the nitrogen, leaving a ketone.[4]
- Formation of an advanced glycation end-product (AGE): the Amadori product is oxidized, most often by transition metal catalysis.
The first two steps in this reaction are both reversible, but the last step is irreversible.
See also
- Fructoselysine, the Amadori product derived from glucose and lysine
References
- ↑ M. Amadori, Atti. reale accad. nazl. Lincei, [6] 2, 337 (1925); [6] 9, 68, 226 (1929); [6] 13, 72 (1931)
- ↑ Strategic Applications of Named Reactions in Organic Synthesis (Paperback) by Laszlo Kurti, BN 0-12-429785-4
- ↑ Isbell, Horace S.; Frush, Harriet L. (1958). "Mutarotation, Hydrolysis, and Rearrangement Reactions of Glycosylamines1". The Journal of Organic Chemistry. 23 (9): 1309. doi:10.1021/jo01103a019.
- 1 2 Berillo, Dmitriy; Natalia Volkova (2014). "Preparation and physicochemical characteristics of cryogel based on gelatin and oxidised dextran". Journal of Materials Science. 49 (14): 4855–4868. Bibcode:2014JMatS..49.4855B. doi:10.1007/s10853-014-8186-3.
External links
- Amadori Rearrangement, PowerPoint presentation detailing the reaction mechanism