Apolipoprotein A1
| View/Edit Human | View/Edit Mouse |
Apolipoprotein A1 is a protein that in humans is encoded by the APOA1 gene.[4][5] It has a specific role in lipid metabolism. The text in a recent report suggested that APOA1 mRNA is regulated by endogenously expressed antisense RNA.[6]
Structure
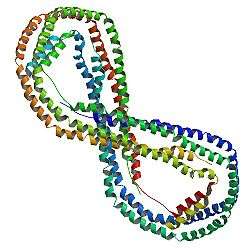
The APOA1 gene is located on the 11th chromosome, with its specific location being 11q23-q24. The gene contains 4 exons.[7] APOA1 encodes a 45.4 kDa protein that is composed of 396 amino acids; 21 peptides have been observed through mass spectrometry data.[8][9]
Function
Apolipoprotein A1 is the major protein component of HDL particles in plasma.
Chylomicrons secreted from the intestinal enterocyte also contain apo A1, but it is quickly transferred to HDL in the bloodstream.[10]
The protein, as a component of HDL particles, enables efflux of fat molecules by accepting fats from within cells (including macrophages within the walls of arteries which have become overloaded with injested fats from oxidized LDL particles) for transport (in the water outside cells) elsewhere, including back to LDL particles or to the liver for excretion.
It is a cofactor for lecithin cholesterolacyltransferase (LCAT) which is responsible for the formation of most plasma cholesteryl esters. Apo A1 was also isolated as a prostacyclin (PGI2) stabilizing factor, and thus may have an anticlotting effect.[11] Defects in the gene encoding it are associated with HDL deficiencies, including Tangier disease, and with systemic non-neuropathic amyloidosis.[7]
ApoA1 is often used as a biomarker for prediction of cardiovascular diseases. The ratio apoB-100/apoA1 (i.e. LDL & larger particles vs. HDL particles), NMR measured Lipoprotein (LDL/HDL) particle ratios even more so,[12] has always had a stronger correlation with myocardial infarction event rates than older methods of measuring lipid transport in the water outside cells.[13]
ApoA1 is routinely measured using immunoassays such as ELISA or nephelometry.
Clinical significance
Activity associated with high HDL-C and protection from heart disease
As a major component of the high-density lipoprotein complex (protective "fat removal" particles), apo A1 helps to clear fats, including cholesterol, from white blood cells within artery walls, making the WBCs less likely to become fat overloaded, transform into foam cells, die and contribute to progressive atheroma. Five of nine men found to carry a mutation (E164X) who were at least 35 years of age had developed premature coronary artery disease.[14] One of four mutants of apo A1 is present in roughly 0.3% of the Japanese population, but is found in 6% of those with low HDL cholesterol levels.
ApoA-1 Milano is a naturally occurring mutant of apo A1, found in a few families in Limone sul Garda, Italy, and, by genetic + church record family tree detective work, traced to a single individual in the 18th century. Described in 1980, it was the first known molecular abnormality of apolipoproteins.[15] Paradoxically, carriers of this mutation have very low HDL-C (HDL-Cholesterol) levels, but no increase in the risk of heart disease, often living to age 100 or older. This unusual observation was what lead Italian investigators to track down what was going on and lead to the discovery of apo A1 Milano (the city, Milano, ~160 km away, in which the researcher's lab was located). Biochemically, apo A1 contains an extra cysteine bridge, causing it to exist as a homodimer or as a heterodimer with apo A-II. However, the enhanced cardioprotective activity of this mutant (which likely depends on fat & cholesterol efflux) cannot easily be replicated by other cysteine mutants.[16]
Recombinant apo A1 Milano dimers formulated into liposomes can reduce atheromas in animal models by up to 30%.[17] Apo A1 Milano has also been shown in small clinical trials to have a statistically significant effect in reducing (reversing) plaque build-up on arterial walls.[18][19]
In human trials the reversal of plaque build-up was measured over the course of five weeks.[18][20]
Novel Haplotypes within apolipoprotein AI-CIII-AIV gene cluster
Lately, two novel susceptibility haplotypes i.e. P2-S2-X1 and P1-S2-X1 have been discovered in ApoAI-CIII-AIV gene cluster on chromosome 11q23, which confer approximately threefold higher risk of coronary heart disease in normal[21] as well as in the patients having non-insulin diabetes mellitus.[22]
Role in other diseases
A G/A polymorphism in the promoter of the apo A1 gene has been associated with the age at which patients presented with Alzheimer disease.[23] Protection from Alzheimer's disease by apo A1 may rely on a synergistic interaction with alpha-tocopherol.[24] Amyloid deposited in the knee following surgery consists largely of apo A1 secreted from chondrocytes (cartilage cells).[25] A wide variety of amyloidosis symptoms are associated with rare Apo A1 mutants.
Apo A-I binds to lipopolysaccharide or endotoxin, and has a major role in the anti-endotoxin function of HDL.[26]
In one study, a decrease in apo A1 levels was detected in schizophrenia patients' CSF, brain and peripheral tissues.[27]
Epistatic impact of apo A1
Apolipoprotein A1 and APOE interact epistatically to modulate triglyceride levels in coronary heart disease patients. Individually, neither apo A1 nor apo E was found to be associated with triglyceride (TG) levels, but pairwise epistasis (additive x additive model) explored their significant synergistic contributions with raised TG levels (P<0.01). [28]
Factors affecting apo A1 activity
Apo A1 production is decreased by calcitriol, and increased by a drug that antagonizes it.[29]
Exercise or statin treatment may cause an increase in HDL-C levels by inducing apo A1 production, but this depends on the G/A promoter polymorphism.[30]
Interactions
Apolipoprotein A1 has been shown to interact with:
Potential binding partners
Apolipoprotein A1 binding precursor, a relative of APOA-1 abbreviated APOA1BP, has a predicted biochemical interaction with Carbohydrate Kinase Domain Containing Protein. The relationship between these two proteins is substantiated by cooccurance across genomes and coexpression.[34] The ortholog of CARKD in E. coli contains a domain not present in any eukaryotic ortholog. This domain has a high sequence identity to APOA1BP. CARKD is a protein of unknown function, and the biochemical basis for this interaction is unknown.
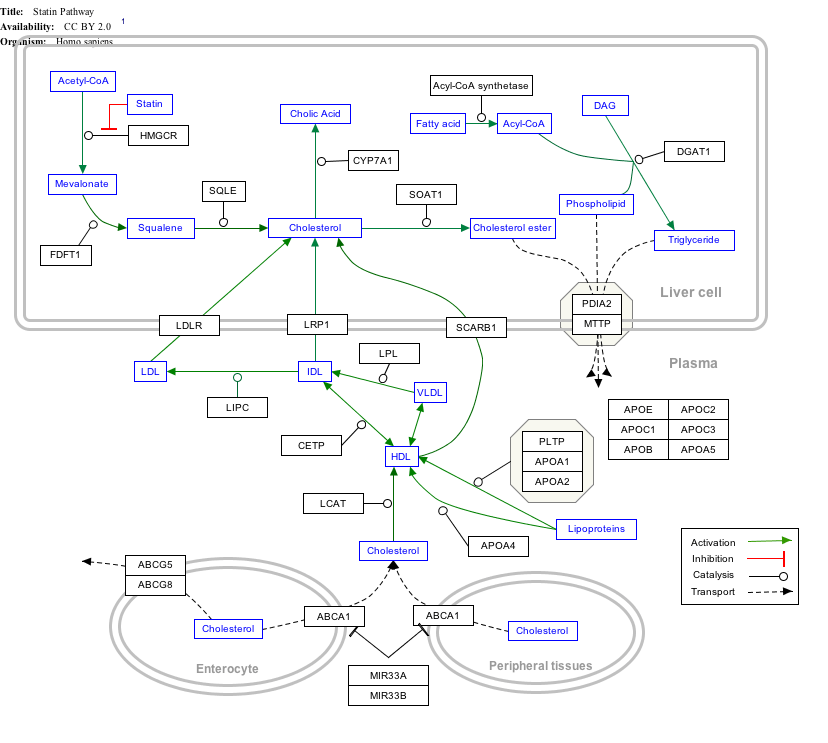
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
Statin Pathway edit
- ↑ The interactive pathway map can be edited at WikiPathways: "Statin_Pathway_WP430".
See also
References
- ↑ "Diseases that are genetically associated with APOA1 view/edit references on wikidata".
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Breslow JL, Ross D, McPherson J, Williams H, Kurnit D, Nussbaum AL, Karathanasis SK, Zannis VI (November 1982). "Isolation and characterization of cDNA clones for human apolipoprotein A1". Proc. Natl. Acad. Sci. U.S.A. 79 (22): 6861–5. doi:10.1073/pnas.79.22.6861. PMC 347233
 . PMID 6294659.
. PMID 6294659. - ↑ Arinami T, Hirano T, Kobayashi K, Yamanouchi Y, Hamaguchi H (June 1990). "Assignment of the apolipoprotein A1 gene to 11q23 based on RFLP in a case with a partial deletion of chromosome 11, del(11)(q23.3----qter)". Hum. Genet. 85 (1): 39–40. doi:10.1007/BF00276323. PMID 1972696.
- ↑ Halley P, Kadakkuzha BM, Faghihi MA, Magistri M, Zeier Z, Khorkova O, Coito C, Hsiao J, Lawrence M, Wahlestedt C (16 January 2014). "Regulation of the Apolipoprotein Gene Cluster by a Long Noncoding RNA". Cell Reports. 6 (1): 222–230. doi:10.1016/j.celrep.2013.12.015. PMID 24388749.
- 1 2 "Entrez Gene: APOA1 apolipoprotein A1".
- ↑ ]Zong NC, Li H, Li H, Lam MP, Jimenez RC, Kim CS, Deng N, Kim AK, Choi JH, Zelaya I, Liem D, Meyer D, Odeberg J, Fang C, Lu HJ, Xu T, Weiss J, Duan H, Uhlen M, Yates JR, Apweiler R, Ge J, Hermjakob H, Ping P (Oct 2013). "Integration of cardiac proteome biology and medicine by a specialized knowledgebase". Circulation Research. 113 (9): 1043–53. doi:10.1161/CIRCRESAHA.113.301151. PMC 4076475
 . PMID 23965338.
. PMID 23965338. - ↑ "Apolipoprotein A-IV". Cardiac Organellar Protein Atlas Knowledgebase (COPaKB).
- ↑ Wasan KM, Brocks DR, Lee SD, Sachs-Barrable K, Thornton SJ (January 2008). "Impact of lipoproteins on the biological activity and disposition of hydrophobic drugs: implications for drug discovery". Nature Reviews Drug Discovery. 7 (1): 84–99. doi:10.1038/nrd2353. PMID 18079757.
- ↑ Yui Y, Aoyama T, Morishita H, Takahashi M, Takatsu Y, Kawai C (1988). "Serum prostacyclin stabilizing factor is identical to apolipoprotein A1 (apo A1). A novel function of apo A1". J. Clin. Invest. 82 (3): 803–7. doi:10.1172/JCI113682. PMC 303586
 . PMID 3047170.
. PMID 3047170. - ↑ https://www.google.com/search?q=LDL-P+vs.+LDL-C+%26+Cardiovascular+Event+Rates
- ↑ McQueen MJ, Hawken S, Wang X, Ounpuu S, Sniderman A, Probstfield J, Steyn K, Sanderson JE, Hasani M, Volkova E, Kazmi K, Yusuf S (2008). "Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study". Lancet. 372 (9634): 224–33. doi:10.1016/S0140-6736(08)61076-4. PMID 18640459.
- ↑ Dastani Z, Dangoisse C, Boucher B, Desbiens K, Krimbou L, Dufour R, Hegele RA, Pajukanta P, Engert JC, Genest J, Marcil M (March 2006). "A novel nonsense apolipoprotein A-I mutation (apoA-I(E136X)) causes low HDL cholesterol in French Canadians". Atherosclerosis. 185 (1): 127–36. doi:10.1016/j.atherosclerosis.2005.05.028. PMID 16023124.
- ↑ Franceschini G, Sirtori M, Gianfranceschi G, Sirtori CR (May 1981). "Relation between the HDL apoproteins and A-I isoproteins in subjects with the AIMilano abnormality". Metab. Clin. Exp. 30 (5): 502–9. doi:10.1016/0026-0495(81)90188-8. PMID 6785551.
- ↑ Zhu X, Wu G, Zeng W, Xue H, Chen B (2005). "Cysteine mutants of human apolipoprotein A-I: a study of secondary structural and functional properties". J. Lipid Res. 46 (6): 1303–11. doi:10.1194/jlr.M400401-JLR200. PMID 15805548.
- ↑ Chiesa G, Sirtori CR (2003). "Apolipoprotein A-I(Milano): current perspectives". Curr. Opin. Lipidol. 14 (2): 159–63. doi:10.1097/00041433-200304000-00007. PMID 12642784.
- 1 2 "Apo A-I-Milano Trial: Where are we now?". Cleveland Clinic. Retrieved 2008-07-26.
- ↑ Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL, Halpern S, Crowe T, Blankenship JC, Kerensky R (November 2003). "Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial". JAMA. 290 (17): 2292–300. doi:10.1001/jama.290.17.2292. PMID 14600188.
- ↑ "Apo A-I Milano". Cedars-Sinai Heart Institute. Archived from the original on 21 December 2007. Retrieved 2008-07-26.
- ↑ Singh P, Singh M, Kaur TP, Grewal SS (September 2007). "A novel haplotype in ApoAI-CIII-AIV gene region is detrimental to Northwest Indians with coronary heart disease". Int. J. Cardiol. 130 (3): e93–5. doi:10.1016/j.ijcard.2007.07.029. PMID 17825930.
- ↑ Singh P, Singh M, Gaur S, Kaur T (June 2007). "The ApoAI-CIII-AIV gene cluster and its relation to lipid levels in type 2 diabetes mellitus and coronary heart disease: determination of a novel susceptible haplotype". Diab Vasc Dis Res. 4 (2): 124–9. doi:10.3132/dvdr.2007.030. PMID 17654446.
- ↑ Vollbach H, Heun R, Morris CM, Edwardson JA, McKeith IG, Jessen F, Schulz A, Maier W, Kölsch H (2005). "APOA1 polymorphism influences risk for early-onset non-familial AD". Ann. Neurol. 58 (3): 436–41. doi:10.1002/ana.20593. PMID 16130094.
- ↑ Maezawa I, Jin LW, Woltjer RL, Maeda N, Martin GM, Montine TJ, Montine KS (2004). "Apolipoprotein E isoforms and apolipoprotein A-I protect from amyloid precursor protein carboxy terminal fragment-associated cytotoxicity". J. Neurochem. 91 (6): 1312–21. doi:10.1111/j.1471-4159.2004.02818.x. PMID 15584908.
- ↑ Solomon A, Murphy CL, Kestler D, Coriu D, Weiss DT, Makovitzky J, Westermark P (2006). "Amyloid contained in the knee joint meniscus is formed from apolipoprotein A-I". Arthritis Rheum. 54 (11): 3545–50. doi:10.1002/art.22201. PMID 17075859.
- ↑ Ma J, Liao XL, Lou B, Wu MP (2004). "Role of apolipoprotein A-I in protecting against endotoxin toxicity". Acta Biochim. Biophys. Sin. (Shanghai). 36 (6): 419–24. doi:10.1093/abbs/36.6.419. PMID 15188057.
- ↑ Huang JT, Wang L, Prabakaran S, Wengenroth M, Lockstone HE, Koethe D, Gerth CW, Gross S, Schreiber D, Lilley K, Wayland M, Oxley D, Leweke FM, Bahn S (2007). "Independent protein-profiling studies show a decrease in apolipoprotein A1 levels in schizophrenia CSF, brain and peripheral tissues". Mol Psychiatry. 13 (12): 1118–28. doi:10.1038/sj.mp.4002108. PMID 17938634.
- ↑ Singh P, Singh M, Kaur T (2008). "Role of apolipoproteins E and A-I: Epistatic villains of triglyceride mediation in coronary heart disease". Int J Cardiol. 134 (3): 410–2. doi:10.1016/j.ijcard.2007.12.102. PMID 18378026.
- ↑ Wehmeier K, Beers A, Haas MJ, Wong NC, Steinmeyer A, Zugel U, Mooradian AD (2005). "Inhibition of apolipoprotein AI gene expression by 1, 25-dihydroxyvitamin D3". Biochim. Biophys. Acta. 1737 (1): 16–26. doi:10.1016/j.bbalip.2005.09.004. PMID 16236546.
- ↑ Lahoz C, Peña R, Mostaza JM, Jiménez J, Subirats E, Pintó X, Taboada M, López-Pastor A (2003). "Apo A-I promoter polymorphism influences basal HDL-cholesterol and its response to pravastatin therapy". Atherosclerosis. 168 (2): 289–95. doi:10.1016/S0021-9150(03)00094-7. PMID 12801612.
- ↑ Fitzgerald ML, Morris AL, Rhee JS, Andersson LP, Mendez AJ, Freeman MW (September 2002). "Naturally occurring mutations in the largest extracellular loops of ABCA1 can disrupt its direct interaction with apolipoprotein A-I". J. Biol. Chem. 277 (36): 33178–87. doi:10.1074/jbc.M204996200. PMID 12084722.
- ↑ Deeg MA, Bierman EL, Cheung MC (March 2001). "GPI-specific phospholipase D associates with an apoA-I- and apoA-IV-containing complex". J. Lipid Res. 42 (3): 442–51. PMID 11254757.
- ↑ Pussinen PJ, Jauhiainen M, Metso J, Pyle LE, Marcel YL, Fidge NH, Ehnholm C (January 1998). "Binding of phospholipid transfer protein (PLTP) to apolipoproteins A-I and A-II: location of a PLTP binding domain in the amino terminal region of apoA-I". J. Lipid Res. 39 (1): 152–61. PMID 9469594.
- ↑ "STRING: Known and Predicted Protein-Protein Interactions".
External links
- Apolipoprotein A-I at the US National Library of Medicine Medical Subject Headings (MeSH)
- Applied Research on Apolipoprotein-A1