Lipoprotein
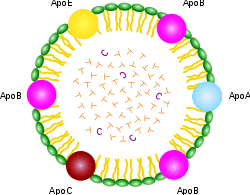
ApoA, ApoB, ApoC, ApoE (apolipoproteins); T (triacylglycerol); C (cholesterol); green (phospholipids)
A Lipoprotein is a biochemical assembly whose purpose is to transport hydrophobic lipids (aka fat) molecules in water, as in blood or ECF. They have a single layer phospholipid and cholesterol outer shell, with the hydrophilic portions oriented outward toward the surrounding water and lipophilic portions of each molecule oriented inwards toward the lipids molecules within the particles. Apolipoproteins are embedded in the membrane, both stabilising the complex and giving it functional identity determining its fate. Thus the complex serves to emulsify the fats. Many enzymes, transporters, structural proteins, antigens, adhesins, and toxins are lipoproteins. Examples include the plasma lipoprotein particles classified under HDL, LDL, IDL, VLDL and ULDL (commonly called chylomicron) lipoproteins, which enable fats to be carried in the blood stream (an example of emulsification), the transmembrane proteins of the mitochondrion and the chloroplast, and bacterial lipoproteins.[1]
Scope
Transmembrane lipoproteins
The lipids are often an essential part of the complex, even if they seem to have no catalytic activity by themselves. To isolate transmembrane lipoproteins from their associated biological membranes, detergents are often needed.
Plasma lipoprotein particles
The role of lipoprotein particles is to transport triacylglycerols (a.k.a. triglycerides) and cholesterol in the blood between all the tissues of the body. The most common being the liver and the adipocytes of adipose tissue. Particles are synthesized in the small intestine and the liver, but interestingly not in the adipocytes.
All cells use and rely on fats and cholesterol as building-blocks to create the multiple membranes that cells use both to control internal water content and internal water-soluble elements and to organize their internal structure and protein enzymatic systems.
The lipoprotein particles have hydrophilic groups of phospholipids, cholesterol, and apoproteins directed outward. Such characteristics make them soluble in the salt water-based blood pool. Triglyceride-fats and cholesteryl esters are carried internally, shielded from the water by the phospholipid monolayer and the apoproteins.
The interaction of the proteins forming the surface of the particles (with enzymes in the blood; with each other; and with specific proteins on the surfaces of cells) determines whether triglycerides and cholesterol will be added to or removed from the lipoprotein transport particles.
Regarding atheroma development and progression as opposed to regression, the key issue has always been cholesterol transport patterns, not cholesterol concentration itself.
Function
The handling of lipoprotein particles in the body is referred to as lipoprotein particle metabolism. It is divided into two pathways, exogenous and endogenous, depending in large part on whether the lipoprotein particles in question are composed chiefly of dietary (exogenous) lipids or whether they originated in the liver (endogenous), through de novo synthesis of triacylglycerols.
The hepatocytes are the main platform for the handling of triacylglycerols and cholesterol; the liver can also store certain amounts of glycogen and triacylglycerols. While adipocytes are the main storage cells for triacylglycerols, they do not produce any lipoproteins.
Exogenous pathway

Bile emulsifies fats contained in the chyme, then pancreatic lipase cleaves triacylglycerol molecules into two fatty acids and one 2-monoacylglycerol. Enterocytes readily absorb these small molecules from the chymus. Inside of the enterocytes, fatty acids and monoacylglycerides are transformed again into triacylglycerides. Then these lipids (i.e. triacylglycerols, phospholipids, cholesterol, and cholesteryl esters) are assembled with apolipoprotein B-48 into nascent chylomicrons. These particles are then secreted into the lacteals in a process that depends heavily on apolipoprotein B-48. As they circulate through the lymphatic vessels, nascent chylomicrons bypass the liver circulation and are drained via the thoracic duct into the bloodstream.
In the blood stream, nascent chylomicron particles interact with HDL particles resulting in HDL donatation of apolipoprotein C-II and apolipoprotein E to the nascent chylomicron. The chylomicron at this stage is then considered mature. Via apolipoprotein C-II, mature chylomicrons activate lipoprotein lipase (LPL), an enzyme on endothelial cells lining the blood vessels. LPL catalyzes the hydrolysis of triacylglycerol (glycerol covalently joined to three fatty acids) that ultimately releases glycerol and fatty acids from the chylomicrons. Glycerol and fatty acids can then be absorbed in peripheral tissues, especially adipose and muscle, for energy and storage.
The hydrolyzed chylomicrons are now called chylomicron remnants. The chylomicron remnants continue circulating the bloodstream until they interact via apolipoprotein E with chylomicron remnant receptors, found chiefly in the liver. This interaction causes the endocytosis of the chylomicron remnants, which are subsequently hydrolyzed within lysosomes. Lysosomal hydrolysis releases glycerol and fatty acids into the cell, which can be used for energy or stored for later use.
Endogenous pathway
The liver is the central platform for the handling of lipids: it is able to store glycerols and fats in its cells, the hepatocytes. Hepatocytes are also able to create triacylglycerols via de novo synthesis. They also produce the bile from cholesterol.
In the hepatocytes, triacylglycerols and cholesteryl esters are assembled with apolipoprotein B-100 to form nascent VLDL particles. Nascent VLDL particles are released into the bloodstream via a process that depends upon apolipoprotein B-100.
In the blood stream, nascent VLDL particles bump with HDL particles; as a result, HDL particles donate apolipoprotein C-II and apolipoprotein E to the nascent VLDL particle; Once loaded with apolipoproteins C-II and E, the nascent VLDL particle is considered mature.
Again, like chylomicrons, VLDL particles circulate and encounter lipoprotein lipase (LPL) expressed on endothelial cells. Apolipoprotein C-II activates LPL, causing hydrolysis of the VLDL particle and the release of glycerol and fatty acids. These products can be absorbed from the blood by peripheral tissues, principally adipose and muscle. The hydrolyzed VLDL particles are now called VLDL remnants or intermediate-density lipoproteins (IDLs). VLDL remnants can circulate and, via an interaction between apolipoprotein E and the remnant receptor, be absorbed by the liver, or they can be further hydrolyzed by hepatic lipase.
Hydrolysis by hepatic lipase releases glycerol and fatty acids, leaving behind IDL remnants, called low-density lipoproteins (LDL), which contain a relatively high cholesterol content[2] (see native LDL structure at 37°C on YouTube). LDL circulates and is absorbed by the liver and peripheral cells. Binding of LDL to its target tissue occurs through an interaction between the LDL receptor and apolipoprotein B-100 on the LDL particle. Absorption occurs through endocytosis, and the internalized LDL particles are hydrolyzed within lysosomes, releasing lipids, chiefly cholesterol.
Classification
By density
Lipoproteins may be classified as follows, listed from larger and less dense to smaller and denser. Lipoproteins are larger and less dense when the fat to protein ratio is increased. They are classified on the basis of electrophoresis and ultracentrifugation.
- Chylomicrons carry triglycerides (fat) from the intestines to the liver, to skeletal muscle, and to adipose tissue.
- Very-low-density lipoproteins (VLDL) carry (newly synthesised) triglycerides from the liver to adipose tissue.
- Intermediate-density lipoproteins (IDL) are intermediate between VLDL and LDL. They are not usually detectable in the blood when fasting.
- Low-density lipoproteins (LDL) carry 3,000 to 6,000 fat molecules (phospholipids, cholesterol, triglycerides, etc.) around the body. LDL particles are sometimes referred to as "bad" lipoprotein because concentrations, dose related, correlate with atherosclerosis progression.
- large buoyant LDL (lb LDL) particles
- small dense LDL (sd LDL) particles
- Lipoprotein(a) is a lipoprotein particle of a certain phenotype
- High-density lipoproteins (HDL) collect fat molecules (phospholipids, cholesterol, triglycerides, etc.) from the body's cells/tissues, and take it back to the liver. HDLs are sometimes referred to as "good" lipoprotein because higher concentrations correlate with low rates of atherosclerosis progression and/or regression.
For young healthy research subjects, ~70 kg, 154 lb, the following applies:
| Density (g/mL) | Class | Diameter (nm) | % protein | % cholesterol | % phospholipid | % triacylglycerol & cholesterol ester |
| >1.063 | HDL | 5–15 | 33 | 30 | 29 | 4 |
| 1.019–1.063 | LDL | 18–28 | 25 | 50 | 21 | 8 |
| 1.006–1.019 | IDL | 25–50 | 18 | 29 | 22 | 31 |
| 0.95–1.006 | VLDL | 30–80 | 10 | 22 | 18 | 50 |
| <0.95 | Chylomicrons | 100-1000 | <2 | 8 | 7 | 84 |
[3] However, this data is not reliable for the general clinical population.
Alpha and beta
It is also possible to classify lipoproteins as "alpha" and "beta", according to the classification of proteins in serum protein electrophoresis. This terminology is sometimes used in describing lipid disorders such as Abetalipoproteinemia.
Lipoprotein(a)
Studies
Atherosclerosis is the leading cause of coronary artery disease, which is the leading cause of mortality in the world.[4] Since the 1980s, many studies have examined possible correlations between the incidence of the disease and plasma lipoprotein particle concentrations in the blood. Hypotheses exist for possible causations. Studies have shown correlation between atherosclerosis and concentrations of particles. Further studies looked for correlations between nutrition and concentration of the distinguishable lipoprotein particles, e.g. whether the ratio of dietary fat raises or lowers levels of LDL particles in the blood. Studies have shown that different phenotypes do exist regarding the amount of particles and reaction to diet composition.
See also
References
- ↑ mrc-lmb.cam.ac.uk
- ↑ Kumar, Vibhor; Butcher, Sarah J.; Öörni, Katariina; Engelhardt, Peter; Heikkonen, Jukka; Kaski, Kimmo; Ala-Korpela, Mika; Kovanen, Petri T.; Schulz, Christian (9 May 2011). "Three-Dimensional cryoEM Reconstruction of Native LDL Particles to 16Å Resolution at Physiological Body Temperature". PLoS ONE. 6 (5): e18841. doi:10.1371/journal.pone.0018841. PMC 3090388
 . PMID 21573056.
. PMID 21573056. - ↑ Biochemistry 2nd Ed. 1995 Garrett & Grisham
- ↑ World Health Organization: The top 10 causes of death
- Further reading
- Lusis, Aldons J; Pajukanta, Päivi. "A treasure trove for lipoprotein biology". Nature Genetics. 40 (2): 129–130. doi:10.1038/ng0208-129. PMID 18227868. including Figure 1 - The primary pathways for the metabolism of human plasma lipoproteins are summarized
External links
- Database of bacterial lipoproteins at mrc-lmb.cam.ac.uk
- Overview and diagram at washington.edu
- Lipoprotein research at the Medical University of Vienna
- Lipoprotein assembly at wisc.edu
- Various types of lipoprotein in Medscape
- Cholesterol, Lipoproteins and the Liver
- Remnant Lipoproteins
- Lipoproteins at the US National Library of Medicine Medical Subject Headings (MeSH)
- Proteolipids at the US National Library of Medicine Medical Subject Headings (MeSH)