Biological functions of nitric oxide
The gas nitric oxide plays a wide variety of roles in biological organisms.
Nitric oxide synthesis
Nitric oxide synthases (NOSs) synthesize the metastable free radical nitric oxide (NO). There are three isoforms of the NOS enzyme: endothelial (eNOS), neuronal (nNOS), and inducible (iNOS) - each with separate functions. The neuronal enzyme (NOS-1) and the endothelial isoform (NOS-3) are calcium-dependent and produce low levels of this gas as a cell signaling molecule. The inducible isoform (NOS-2) is calcium-independent and produces large amounts of gas that can be cytotoxic. NOS oxidizes the guanidine group of L-arginine in a process that consumes five electrons and results in the formation of NO with stoichiometric formation of L-citrulline. The process involves the oxidation of NADPH and the reduction of molecular oxygen. The transformation occurs at a catalytic site adjacent to a specific binding site of L-arginine.[1] NO is an important regulator and mediator of numerous processes in the nervous, immune, and cardiovascular systems. These include vascular smooth muscle relaxation, resulting in arterial vasodilation and increasing blood flow.[2] NO is also a neurotransmitter and has been associated with neuronal activity and various functions such as avoidance learning. NO also partially mediates macrophage cytotoxicity against microbes and tumor cells. Besides mediating normal functions, NO is implicated in pathophysiologic states as diverse as septic shock, hypertension, stroke, and neurodegenerative diseases.[3]
Non-enzymatic sources of NO
Currently, exogenous NO sources constitute a powerful way to supplement NO when the body cannot generate enough for normal biological functions. So, recent developments of novel NO donors, NO releasing devices, and innovative improvements to current NO donors have been investigated.[4] It should be noted that certain endogenous compounds can act as NO-donors or elicit NO-like reactions in vivo. Prominent examples are S-nitrosothiols, certain organic nitrates, nitrosylated metal complexes, dinitrosyl-iron complexes (DNIC), and even nitrite anions (NO2− ) under hypoxic conditions [5][6]
Vasodilation
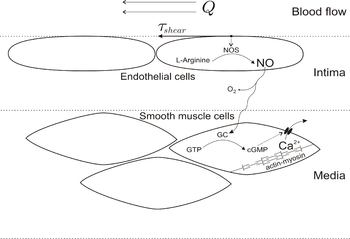
Nitric oxide (NO) is of critical importance as a mediator of vasodilation in blood vessels. It is induced by several factors, and once synthesized by eNOS it results in phosphorylation of several proteins that cause smooth muscle relaxation.[2] The vasodilatory actions of nitric oxide play a key role in renal control of extracellular fluid homeostasis and is essential for the regulation of blood flow and blood pressure.[7] NO also plays a role in erection of the penis and clitoris.[8]
Induction
Platelet-derived factors, shear stress, acetylcholine, and cytokines stimulate the production of NO by endothelial nitric oxide synthase (eNOS). eNOS synthesizes NO from the terminal guanidine-nitrogen of L-arginine and oxygen and yields citrulline as a byproduct. NO production by eNOS is dependent on calcium-calmodulin and other cofactors.
Phosphorylation
NO, a highly reactive free radical, diffuses into the smooth muscle cells of the blood vessel and interacts with soluble guanylate cyclase. Nitric oxide stimulates the soluble guanylate cyclase to generate the second messenger cyclic GMP (3’,5’ guanosine monophosphate) from guanosine triphosphate (GTP). The soluble cGMP activates cyclic nucleotide-dependent protein kinase G (PKG or cGKI). PKG is a kinase that phosphorylates a number of proteins that regulate calcium concentrations and calcium sensitization, hyperpolarizes cell through potassium channels, and causes actin filament and myosin dynamic alterations that result in smooth muscle relaxation. (See the article on smooth muscles).[9]
Penile erection
The vasodilatatory effect of NO, in turn, also plays a role in development and maintenance of penile erection. Vasodilation of blood vessels supplying the corpus cavernosum results in more blood flowing in and, hence, erection. This is the biological basis of sildenafil (Viagra), which works to inhibit the enzyme phosphodiesterase PDE5, which lowers the cGMP concentration by converting it back to GMP.
Immune system
Macrophages, certain cells of the immune system, produce nitric oxide in order to kill invading bacteria. In this case, the nitric oxide synthase is inducible NOS.
Under certain conditions, this can backfire: Fulminant infection (sepsis) causes excess production of nitric oxide by macrophages, leading to vasodilatation (widening of blood vessels), probably one of the main causes of hypotension (low blood pressure) in sepsis. The inducible isoform of nitric oxide synthase is expressed and produces cytotoxic levels of nitric oxide.
Neurotransmission
Nitric oxide also serves as a neurotransmitter between nerve cells, part of its general role in redox signaling. Unlike most other neurotransmitters that transmit information only from a presynaptic to a postsynaptic neuron, the small, uncharged, and fat-soluble nitric oxide molecule can diffuse widely and readily into cells. Thus, it can act on several nearby neurons, even on those not connected by a synapse. At the same time, the short half-life of NO means that such action will be restricted to a limited area, without the necessity for enzymatic breakdown or cellular reuptake. NO is also highly reactive with other free radicals, lipids, and proteins.
NO-cGMP cascade is involved in learning and memory through the maintenance of long-term potentiation (LTP).[10][11]
Nitric oxide is an important non-adrenergic, non-cholinergic (NANC) neurotransmitter in various parts of the gastrointestinal tract. It causes relaxation of the gastrointestinal smooth muscle. In the stomach, it increases the capacity of the fundus to store food and fluids.
Other functions
Dietary nitrate is also an important source of nitric oxide in mammals. Green, leafy vegetables and some root vegetables (such as beetroot) have high concentrations of nitrate.[12] When eaten and absorbed into the bloodstream, nitrate is concentrated in saliva (about 10-fold) and is reduced to nitrite on the surface of the tongue by a biofilm of commensal facultative anaerobic bacteria.[13] This nitrite is swallowed and reacts with acid and reducing substances in the stomach (such as ascorbate) to produce high concentrations of nitric oxide. The purpose of this mechanism to create NO is thought to be both sterilization of swallowed food, to prevent food poisoning, and to maintain gastric mucosal blood flow.[14]
A similar mechanism is thought to protect the skin from fungal infections, where nitrate in sweat is reduced to nitrite by skin commensal organisms and then to NO on the slightly acidic skin surface. In alternative fashion, nitrite anions on sun-exposed skin may be photolyzed to free nitric oxide radicals by UVA in sunlight.[15] This mechanism may elicit significant changes to the systemic blood circulation in humans and exploited for therapeutic purposes[16]
Nitric oxide also acts on cardiac muscle to decrease contractility and heart rate. NO contributes to the regulation of cardiac contractility. Emerging evidence suggests that coronary artery disease (CAD) is related to defects in generation or action of NO.[17] Reduced levels of exhaled NO have been associated with exposure to traffic related air pollution.[18] The bacterium Deinococcus radiodurans can withstand extreme levels of radioactivity and other stresses. In 2009 it was reported that nitric oxide plays an important role in this bacteria's recovery from radiation exposure: The gas is required for division and proliferation after DNA damage has been repaired. A gene that increases nitric oxide production after UV radiation was described, and in the absence of this gene the bacteria were still able to repair DNA damage, but would not grow.[19]
Pathology
People with diabetes usually have lower levels of nitric oxide than patients without diabetes.[20] Diminished supply of nitric oxide can lead to vascular damage, such as endothelial dysfunction and vascular inflammation. Vascular damage can lead to decreased blood flow to the extremities, causing the diabetic patient to be more likely to develop Neuropathy and non-healing ulcers, and to be at a greater risk for lower limb amputation.
Pharmaceutical analogs
Nitroglycerin, amyl nitrite, "poppers" (isobutyl nitrite or similar), and other nitrite derivatives are used in the treatment of heart disease: The compounds are converted to nitric oxide (by a process that is not completely understood), which in turn dilates the coronary artery (blood vessels around the heart), thereby increasing its blood supply. These drugs, however, are predominantly venodilators, dilating peripheral veins and hence reducing venous return and preload to the heart. This reduces the oxygen requirement of the myocardium and subsequently lessens the anginal pain felt with myocardial ischemia.[21]
Effects on plants
Numerous important discoveries about nitric oxide function within plants starting in the 1990s have made it clear that nitric oxide is an important signaling compound in plants.[22] It is involved in such diverse functions as regulation of defense mechanisms in plant-pathogen interaction, promotion of the plant hypersensitive response, symbiosis (for example, with organisms in nitrogen-fixing root nodules), development of lateral and adventitious roots and root hairs, and control of stomatal opening. Nitric oxide is known to be produced by cellular organelles, including mitochondria, peroxisomes, and chloroplasts. It plays a role in antioxidant and reactive oxygen species responses.[23] Nitric oxide interactions have been found within signaling pathways of important plant hormones such as auxin[24] and cytokinin.[25] These recent discoveries are stimulating new research into nitric oxide's role within plants.
Atmospheric nitric oxide can enter the stomates of most vascular species, and can have effects ranging from leaf blemishing, to stunting of growth, to necrosis.[26]
Discovery
Endothelium-derived relaxing factor was originally the name given to several proposed factors causing vasodilation. The major endothelial-derived relaxing factor was later discovered to be nitric oxide (NO).
The discovery of the biological functions of nitric oxide in the 1980s was unexpected. Nitric oxide was named "Molecule of the Year" in 1992 by the journal Science, a Nitric Oxide Society was founded, and a scientific journal devoted entirely to nitric oxide was established. The Nobel Prize in Physiology or Medicine in 1998 was awarded to Ferid Murad, Robert F. Furchgott, and Louis Ignarro for the discovery of the signalling properties of nitric oxide. Salvador Moncada also identified EDRF as NO molecule but did not share the Nobel Prize primarily due to the Nobel policy of honoring only three discoverers. It is estimated that yearly approximately 3,000 scientific articles are published on the biological roles of nitric oxide.
References
- ↑ Ignarro L.J. (2001): Nitric Oxide. A Novel Signal Transduction Mechanism For Transcellular Communication; 16: 477- 483.
- 1 2 Weller, Richard, Could the sun be good for your heart? TedxGlasgow March 2012, posted January 2013
- ↑ Davies, S.A., Stewart, E.J., Huesmaan, G.R and Skaer, N. J. (1997): Neuropeptide stimulation of the nitric oxide signalling pathway in Drosophila melanogaster Malpighian tubules. Am. J. Physiol..; 273, R823-827.
- ↑ Hou, Y.C.; Janczuk, A.; Wang, P.G. (1999). "Current trends in the development of nitric oxide donors". Curr. Pharm. Des. 5 (6): 417–471. PMID 10390607.
- ↑ Radicals for life: The various forms of nitric oxide. E. van Faassen and A. Vanin, eds. Elsevier, Amsterdam 2007. ISBN 978-0-444-52236-8.
- ↑ Nitrite as regulator of hypoxic signaling in mammalian physiology. Med Res Rev 29, 2009, 683 - 741
- ↑ Yoon, Y.; Song, U.; Hong, S.H.; Kim, J.Q. (2000). "Plasma nitric oxide concentration and nitric oxide synthase gene polymorphism in coronary artery disease". Clinic. Chem. 46 (10): 1626–1630.
- ↑ Gragasin, S.; Michelakis, D.; Hogan, A.; Moudgil, R.; Hashimoto, K.; Wu, X.; Bonnet, S.; Haromy, A.; Archer, L. (Sep 2004). "The neurovascular mechanism of clitoral erection: nitric oxide and cGMP-stimulated activation of BKCa channels" (Free full text). The FASEB Journal. 18 (12): 1382–1391. doi:10.1096/fj.04-1978com. ISSN 0892-6638. PMID 15333581.
- ↑ Tanaka, Y.; Tang, G.; Takizawa, K.; Otsuka, K.; Eghbali, M.; Song, M.; Nishimaru, K.; Shigenobu, K.; Koike, K.; Stefani, E.; Toro, L. (2005). "Kv Channels Contribute to Nitric Oxide- and Atrial Natriuretic Peptide-Induced Relaxation of a Rat Conduit Artery". Journal of Pharmacology and Experimental Therapeutics. 317 (1): 341–354. doi:10.1124/jpet.105.096115. PMID 16394199.
- ↑ Hopper, RA; Garthwaite, J (2006). "Tonic and phasic nitric oxide signals in hippocampal long-term potentiation.". Journal of Neuroscience. 26 (45): 11513–21. doi:10.1523/JNEUROSCI.2259-06.2006. PMID 17093072.
- ↑ Taqatqeh, F; Mergia, E; Neitz, A; Eysel, UT; Koesling, D; Mittmann, T (2009). "More than a retrograde messenger: nitric oxide needs two cGMP pathways to induce hippocampal long-term potentiation.". Journal of Neuroscience. 29 (29): 9344–50. doi:10.1523/JNEUROSCI.1902-09.2009. PMID 19625524.
- ↑ http://www.berkeleytest.com/plant-based.html
- ↑ Lundberg, JO; Eddie Weitzberg, E; Gladwin, MT (2008). "The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics". Nature Reviews Drug Discovery. 7: 156–167. doi:10.1038/nrd2466.
- ↑ Green, SJ (1995). "Nitric oxide in mucosal immunity". Nature Medicine. 1 (6): 515–517. doi:10.1038/nm0695-515.
- ↑ Suschek, C.; Opländer, C. (2010). "Nonenzymatic NO production in human skin: Effect of UVA on cutaneous NO stores". Nitric Oxide. 22: 120–135. doi:10.1016/j.niox.2009.10.006.
- ↑ Opländer, C.; et al. (2012). "Dermal application of nitric oxide in vivo: Kinetics, biological responses and therapeutic potential in humans". Clin Pharmacol Ther. 91: 1074–1082. doi:10.1038/clpt.2011.366.
- ↑ Navin, K.T.; Toshio, H.A.; Daigo, S.I.; Hatsuyo, K.; Hisako, M.; Taku, T.S.; Akihisa, A. (2002). "Anti-Atherosclerotic Effect of -Blocker with Nitric Oxide–Releasing Action on the Severe Atherosclerosis". J. Cardiovascular Pharmacology. 39 (2): 298–309. doi:10.1097/00005344-200202000-00017.
- ↑ Jacobs, L; Nawrot, Tim S; De Geus, Bas; Meeusen, Romain; Degraeuwe, Bart; Bernard, Alfred; Sughis, Muhammad; Nemery, Benoit; Panis, Luc (Oct 2010). "Subclinical responses in healthy cyclists briefly exposed to traffic-related air pollution". Environmental Health. 9 (64): 64. doi:10.1186/1476-069X-9-64. PMC 2984475
 . PMID 20973949.
. PMID 20973949. - ↑ Krishna Ramanujan (October 19, 2009). "Research reveals key to world's toughest organism". Physorg.com
- ↑ nfb University Studies - Nitric Oxide Holds Promise for Diabetes
- ↑ Australian Medicines Handbook, July 2008
- ↑ Mur, L. A., Mandon, J., Persijn, S., Cristescu, S. M., Moshkov, I. E., Novikova, G. V., ... & Gupta, K. J. (2013). Nitric oxide in plants: an assessment of the current state of knowledge. AoB PLANTS.doi:10.1093/aobpla/pls052
- ↑ Verma, K., Mehta, S. K., & Shekhawat, G. S. (2013). Nitric oxide (NO) counteracts cadmium induced cytotoxic processes mediated by reactive oxygen species (ROS) in Brassica juncea: cross-talk between ROS, NO and antioxidant responses. BioMetals: an international journal on the role of metal ions in biology, biochemistry, and medicine.
- ↑ Terrile, M. C., París, R., Calderón‐Villalobos, L. I., Iglesias, M. J., Lamattina, L., Estelle, M., & Casalongué, C. A. (2012). Nitric oxide influences auxin signaling through S‐nitrosylation of the Arabidopsis TRANSPORT INHIBITOR RESPONSE 1 auxin receptor. The Plant Journal.
- ↑ Liu, W. Z.; Kong, D. D.; Gu, X. X.; Gao, H. B.; Wang, J. Z.; Xia, M.; He, Y. K. (2013). "Cytokinins can act as suppressors of nitric oxide in Arabidopsis". Proceedings of the National Academy of Sciences. 110 (4): 1548–1553. doi:10.1073/pnas.1213235110.
- ↑ C.Michael Hogan. 2010. "Abiotic factor" Archived June 8, 2013, at the Wayback Machine.. Encyclopedia of Earth. eds Emily Monosson and C. Cleveland. National Council for Science and the Environment. Washington DC
External links
- R.F. Furchgott's website: The Nature of the Endothelium-Derived Relaxing Factor
- Assessing the Potential of Nitric Oxide and the Diabetic Foot
- Nitric Oxide gas effects on body