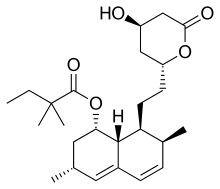
Simvastatin
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˈsɪmvəstætᵻn/ |
| Trade names | Zocor, other |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a692030 |
| License data |
|
| Pregnancy category | |
| Routes of administration | Oral |
| ATC code | C10AA01 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 5% |
| Protein binding | 95% |
| Metabolism | Hepatic (CYP3A4) |
| Biological half-life | 2 hours for simvastatin and 1.9 hours for simvastatin acid |
| Excretion | Renal 13%, faecal 60% |
| Identifiers | |
| |
| CAS Number |
79902-63-9 |
| PubChem (CID) | 54454 |
| IUPHAR/BPS | 2955 |
| DrugBank |
DB00641 |
| ChemSpider |
49179 |
| UNII |
AGG2FN16EV |
| KEGG |
D00434 |
| ChEBI |
CHEBI:9150 |
| ChEMBL |
CHEMBL1064 |
| ECHA InfoCard | 100.115.749 |
| Chemical and physical data | |
| Formula | C25H38O5 |
| Molar mass | 418.566 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Simvastatin, marketed under the trade name Zocor among others, is a lipid-lowering medication.[1] It is used along with exercise, diet, and weight loss to decrease elevated lipid (fat) levels. It is also used to decrease the risk of heart problems in those at high risk. It is taken by mouth.[1]
Serious side effects may include muscle breakdown, liver problems, and increased blood sugar levels. Common side effects include constipation, headaches, and nausea. A lower dose may be needed in people with kidney problems.[1] There is evidence of harm to unborn babies when taken during pregnancy[1][2] and it should not be used by those who are breastfeeding. It is in the statin class of medications and works by decreasing the manufacture of cholesterol by the liver.[1]
Simvastatin was developed by Merck and came into medical use in 1992.[3] It is on the World Health Organization's List of Essential Medicines, the most important medications needed in a basic health system.[4] It is available as a generic medication.[1] The wholesale cost in the developing world is 0.01 to 0.12 USD per day as of 2014.[5] In the United States it costs between 0.50 and 1.00 USD per day.[1] Simvastatin is made from the fungus Aspergillus terreus.[3]
Medical uses
The primary uses of simvastatin are to treat dyslipidemia and to prevent atherosclerosis-related complications such as stroke and heart attacks in those who are at high risk.[1] It is recommended to be used as an addition to a low cholesterol diet.[1]
In the Scandinavian Simvastatin Survival Study (a placebo-controlled, randomized clinical trial of 5 years duration), simvastatin reduced overall mortality in people with existing cardiovascular disease and high LDL cholesterol by 30% and reduced cardiovascular mortality by 42%. The risks of heart attack, stroke, or needing a coronary revascularization procedure were reduced by 37%, 28%, and 37% respectively.[6]
The Heart Protection Study evaluated the effects of simvastatin in people with risk factors including existing cardiovascular disease, diabetes, or stroke but having relatively low LDL cholesterol. In this trial, which lasted 5.4 years, overall mortality was reduced by 13% and cardiovascular mortality was reduced by 18%. People receiving simvastatin experienced 38% fewer non-fatal heart attacks and 25% fewer strokes.[7]
Contraindications
Simvastatin is contraindicated with pregnancy, breastfeeding, and liver disease.[8] Pregnancy must be avoided while on simvastatin due to potentially severe birth defects. Patients cannot breastfeed while on simvastatin due to potentially disrupting the infant's lipid metabolism.[9] High doses of simvastatin are also contraindicated with the widely used antihypertensive amlodipine.[10] A lower dose is also recommended in people taking the calcium channel blockers, verapamil and diltiazem, as well as those taking amiodarone.[11]
Adverse effects
Common side effects (>1% incidence) may include indigestion and eczema. Rare side effects include joint pain, memory loss, and muscle cramps.[12] Cholestatic hepatitis, hepatic cirrhosis, rhabdomyolysis (destruction of muscles and blockade of renal system), and myositis have been reported in patients receiving the drug chronically.[13] Serious allergic reactions to simvastatin are rare.[8] If the following signs of a serious allergic reaction occur, seek medical attention immediately: rash, hoarsness itching/swelling, dizziness, or difficulty swallowing/breathing.[8]
A type of DNA variant known as a single nucleotide polymorphism (SNP) may help predict individuals prone to developing myopathy when taking simvastatin; a study ultimately including 32,000 patients concluded the carriers of one or two risk alleles of a particular SNP, rs4149056,[14] were at a five-fold or 16-fold increased risk, respectively.[15] In 2012, the Clinical Pharmacogenetics Implementation Consortium has released guidelines regarding the use of rs4149056 genotype in guiding dosing of simvastatin[16] and updated the guideline in 2014.[17]
In March 2012, the FDA updated its guidance for statin users to address reports of memory loss, liver damage, increased blood sugar, development of type 2 diabetes, and muscle injury.[18] The new guidance indicates:
- FDA has found that liver injury associated with statin use is rare but can occur.
- The reports about memory loss, forgetfulness, and confusion span all statin products and all age groups. The FDA says these experiences are rare but that those affected often report feeling “fuzzy” or unfocused in their thinking.
- A small increased risk of raised blood sugar levels and the development of type 2 diabetes have been reported with the use of statins.
- Some drugs interact with statins in a way that increases the risk of muscle injury called myopathy, characterized by unexplained muscle weakness or pain.
On March 19, 2010, the FDA issued another statement regarding simvastatin, saying it increases the risk of muscle injury (myopathy) when taken at high doses or at lower doses in combination with other drugs.[19] The highest dose rate causes muscle damage in 610 of every 10,000 people in contrast to a lower dose, which causes muscle damage in eight of 10,000 people.[20] The FDA warning, released again on June 8, 2011, suggested that high dose "simvastatin should be used only in patients who have been taking this dose for 12 months or more without evidence of muscle injury" and that it "should not be started in new patients, including patients already taking lower doses of the drug."[21]
Interactions
Simvastatin has important interactions with grapefruit juice and other drugs, including some that are commonly used for the treatment of cardiovascular disease. These interactions are clinically important because increasing simvastatin serum levels above those normally provided by the maximum recommended dose increases the risk of muscle damage, including the otherwise rare and potentially fatal side effect of rhabdomyolysis.[22]
Consuming large amounts of grapefruit juice increases serum levels of simvastatin by up to three-fold, increasing the risk of side effects.[23][24][25][26] The FDA recommends that people taking statins should avoid consuming more than a quart of grapefruit juice per day.[22]
Simvastatin also interacts with other drugs, including some used to treat cardiovascular problems. It should not be taken by people who are also taking the antifungal drugs fluconazole, itraconazole, or posaconazole; the antibiotics erythromycin, clarithromycin, or telithromycin; HIV protease inhibitors; the antidepressant nefazodone; the cardiovascular drug gemfibrozil; the immunosuppressant ciclosporin, or the endometriosis drug danazol. Reduced maximum doses of simvastatin apply for patients taking certain other drugs, including the cardiovascular drugs verapamil, diltiazem, amiodarone, amlodipine, and ranolazine.[22][27]
Pharmacology
All statins act by inhibiting 3-hydroxy-3-methylglutaryl (HMG) coenzyme A reductase. HMG-CoA reductase, the rate-limiting enzyme of the HMG-CoA reductase pathway, the metabolic pathway responsible for the endogenous production of cholesterol. Statins are more effective than other lipid-regulating drugs at lowering LDL-cholesterol concentration, but they are less effective than the fibrates in reducing triglyceride concentration. However, statins reduce cardiovascular disease events and total mortality irrespective of the initial cholesterol concentration. This is a major piece of evidence that statins work in another way than the lowering of cholesterol (called pleiotropic effects).[28]
The drug is in the form of an inactive lactone that is hydrolyzed after ingestion to produce the active agent. It is a white, nonhygroscopic, crystalline powder that is practically insoluble in water, and freely soluble in chloroform, methanol, and ethanol.
Simvastatin is an effective lipid-lowering drug that can decrease low density lipoprotein (LDL) levels by up to 50%.
History
The development of simvastatin was closely linked with lovastatin. Biochemist Jesse Huff and his colleagues at Merck began researching the biosynthesis of cholesterol in the early 1950s.[29] In 1956, mevalonic acid was isolated from a yeast extract by Karl Folkers, Carl Hoffman, and others at Merck, while Huff and his associates confirmed that mevalonic acid was an intermediate in cholesterol biosynthesis. In 1959, the HMG-CoA reductase enzyme (a major contributor of internal cholesterol production) was discovered by researchers at the Max Planck Institute. This discovery encouraged scientists worldwide to find an effective inhibitor of this enzyme.
By 1976, Akira Endo had isolated the first inhibitor, mevastatin, from the fungus Penicillium citrinium in Sankyo, Japan.[30] In 1979, Hoffman and colleagues isolated lovastatin from a strain of the fungus Aspergillus terreus. While developing and researching lovastatin, Merck scientists synthetically derived a more potent HMG-CoA reductase inhibitor from a fermentation product of A. terreus, which was designated MK-733 (later to be named simvastatin).[31]
In 1994, publication of the results of the Scandinavian Simvastatin Survival Study (4S) provided the first unequivocal evidence that lowering LDL cholesterol via statin treatment reduces cardiovascular events and overall mortality. A total of 4,444 people with coronary heart disease 5.5 to 8.0 mmol/L were randomized to simvastatin treatment or placebo and followed for an average of 5 years. Compared to the placebo group, those treated with simvastatin experienced a 30% decrease in overall mortality, a 42% reduction in coronary death, a 34% reduction in major coronary events, and a 37% reduction in revascularization procedures.[32]
Society and culture
Economics
Simvastatin was introduced in the late 1980s, and since 2006 in many countries, it is available as a generic preparation. This has led to a decrease of the price of most statin drugs, and a reappraisal of the health economics of preventive statin treatment. In the UK in 2008, the typical per-patient cost to the NHS of simvastatin was about £1.50 per month.[33] (40 mg/day costs UK NHS £1.37/month in 2012[34])
Prior to losing U.S. patent protection, simvastatin was Merck & Co.'s largest-selling drug and second-largest-selling cholesterol lowering drug in the world. In 2005, recorded US$3.1 billion of sales in the US and US$4.4 billion worldwide.[35]
Zocor had an original patent expiry date of January 2006, but was extended by the United States Patent Trademark Office (PTO) to expire on June 23, 2006. The PTO granted the patent extension after Merck submitted data from studies of the drug’s positive effect on children. In the UK, the patent for simvastatin had expired by 2004.
In the UK, simvastatin is the most prescribed medication in the community, with 39.9 million items dispensed in 2013. This compares to 30.9 million items for aspirin, and 27.7 million for levothyroxine sodium, the second and third most prescribed drugs in the UK in 2013.[36]
Marketing
Simvastatin was initially marketed by Merck & Co under the trade name Zocor, but is available generically in most countries following the patent expiry. A combination of simvastatin along with ezetimibe is sold under the brand name Vytorin and is jointly marketed by Merck and Schering-Plough.
Brand names include Zocor, Zocor Heart Pro, marketed by the pharmaceutical company Merck & Co. Simlup, Simvotin, Simcard [India], Denan (Germany), Liponorm, Sinvacor, Sivastin (Italy), Lipovas (Japan), Lodales (France), Zocord (Austria and Sweden), Zimstat, Simvahexal (Australia), Lipex (Australia and New Zealand), Simvastatin-Teva, Simvacor, Simvaxon, Simovil (Israel), available in Thailand under the brand Bestatin manufactured by Berlin Pharmaceutical Industry Co Ltd and others.
The primary U.S. patent for Zocor expired on June 23, 2006. Ranbaxy Laboratories (at the 80-mg strength) and Teva Pharmaceutical Industries through its Ivax Pharmaceuticals unit (at all other strengths) were given approval by the FDA to manufacture and sell simvastatin as a generic drug with 180-day exclusivity. Dr. Reddy's Laboratories also has a license from Merck & Co. to sell simvastatin as an authorized generic drug.
See also
References
- 1 2 3 4 5 6 7 8 9 "Simvastatin". The American Society of Health-System Pharmacists. Retrieved Jan 8, 2015.
- ↑ "Prescribing medicines in pregnancy database". Australian Government. 3 March 2014. Retrieved 22 April 2014.
- 1 2 Cechinel-Filho, Valdir (2012). Plant bioactives and drug discovery : principles, practice, and perspectives. Hoboken, N.J.: John Wiley & Sons. p. 104. ISBN 9780470582268.
- ↑ "19th WHO Model List of Essential Medicines (April 2015)" (PDF). WHO. April 2015. Retrieved May 10, 2015.
- ↑ "Simvastatin". International Drug Price Indicator Guide. Retrieved 28 November 2015.
- ↑ "www.accessdata.fda.gov" (PDF).
- ↑ "www.accessdata.fda.gov" (PDF).
- 1 2 3 "Zocor". RxList. 14 November 2012. Retrieved 1 December 2012.
- ↑ "Simvastatin". LactMed. U.S. National Library of Medicine. Retrieved 1 December 2012.
- ↑ "FDA Drug Safety Communication: New restrictions, contraindications, and dose limitations for Zocor (simvastatin) to reduce the risk of muscle injury". Federal Drug Administration. Retrieved 15 January 2013.
- ↑ "Simvastatin: updated advice on drug interactions - updated contraindications". Retrieved 3 Nov 2015.
- ↑ "www.accessdata.fda.gov" (PDF).
- ↑ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 1431–3.
- ↑ rs4149056
- ↑ SEARCH Collaborative Group,, Group; Link E; Parish S; Armitage J; Bowman L; Heath S; Matsuda F; Gut I; Lathrop M; Collins R (2008). "SLCO1B1 variants and statin-induced myopathy--a genomewide study" (pdf). N Engl J Med. 359 (8): 789–99. doi:10.1056/NEJMoa0801936. PMID 18650507.
- ↑ Ramsey, LB; Johnson, SG; Caudle, KE; Haidar, CE; Voora, D; Wilke, RA; Maxwell, WD; McLeod, HL; Krauss, RM; Roden, DM; Feng, Q; Cooper-DeHoff, RM; Gong, L; Klein, TE; Wadelius, M; Niemi, M (October 2014). "The clinical pharmacogenetics implementation consortium guideline for SLCO1B1 and simvastatin-induced myopathy: 2014 update.". Clinical pharmacology and therapeutics. 96 (4): 423–8. doi:10.1038/clpt.2014.125. PMID 24918167.
- ↑ Wilke, RA; Ramsey, LB; Johnson, SG; Maxwell, WD; McLeod, HL; Voora, D; Krauss, RM; Roden, DM; Feng, Q; Cooper-Dehoff, RM; Gong, L; Klein, TE; Wadelius, M; Niemi, M; Clinical Pharmacogenomics Implementation Consortium, (CPIC) (July 2012). "The clinical pharmacogenomics implementation consortium: CPIC guideline for SLCO1B1 and simvastatin-induced myopathy.". Clinical pharmacology and therapeutics. 92 (1): 112–7. doi:10.1038/clpt.2012.57. PMID 22617227.
- ↑ "FDA Expands Advice on Statin Risks". FDA. Retrieved 12 July 2012.
- ↑ http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm204882.htm
- ↑ Steve Sternberg (2011-06-09). "Simvastatin can damage muscles in high doses". USA Today. Retrieved 2011-06-09.
The cholesterol-lowering drug simvastatin can cause severe muscle damage and should not be prescribed in high doses to patients who have taken it for less than a year or in any dose to people taking certain drugs, health officials said Tuesday. . . . Research has shown that the highest dose of simvastatin, 80 milligrams, causes muscle damage in 61 of every 1,000 patients, far higher than the eight-per-10,000 rate in patients taking a 40-milligram dose, Rosenblatt says.
- ↑ "FDA Drug Safety Communication: New restrictions, contraindications, and dose limitations for Zocor (simvastatin) to reduce the risk of muscle injury". U.S. Food and Drug Administration (FDA). June 8, 2011. Retrieved 2011-06-09.
The U.S. Food and Drug Administration (FDA) is recommending limiting the use of the highest approved dose of the cholesterol-lowering medication, simvastatin (80 mg) because of increased risk of muscle damage.
- 1 2 3 "FDA Drug Safety Communication: New restrictions, contraindications, and dose limitations for Zocor (simvastatin) to reduce the risk of muscle injury".
- ↑ "Grapefruit juice-simvastatin interaction: effect on serum concentrations of simvastatin, simvastatin acid, and HMG-CoA reductase inhibitors". November 1998. Retrieved 25 September 2013.
- ↑ "Effects of regular consumption of grapefruit juice on the pharmacokinetics of simvastatin". July 2004. Retrieved 25 September 2013.
- ↑ "Cholesterol-lowering medicines, statins - Interactions". NHS. 16 April 2012. Retrieved 25 September 2013.
- ↑ "Duration of effect of grapefruit juice on the pharmacokinetics of the CYP3A4 substrate simvastatin". October 2004. Retrieved 25 September 2013.
- ↑ "Information on Simvastatin/Amiodarone". Food and Drug Administration (FDA). Retrieved 2008-09-21.
- ↑ Pedersen, TR (2010). "Pleiotropic effects of statins: Evidence against benefits beyond LDL-cholesterol lowering". American Journal of Cardiovascular Drugs. 10 Suppl 1: 10–7. doi:10.2165/1158822-S0-000000000-00000. PMID 21391729.
- ↑ Jie Jack Li (2009). Triumph of the Heart : The Story of Statins. Oxford University Press. p. 59. ISBN 0198043511.
- ↑ Liao JK, Laufs U; Laufs (2005). "Pleiotropic effects of statins". Annu. Rev. Pharmacol. Toxicol. 45: 89–118. doi:10.1146/annurev.pharmtox.45.120403.095748. PMC 2694580
 . PMID 15822172.
. PMID 15822172. - ↑ Olivia Williams; Anne-Marie Jacks; Jim Davis; Sabrina Martinez (1998). "Case 10: Merck(A): Mevacor". In Allan Afuah. Innovation Management - Strategies, Implementation, and Profits. Oxford University Press. ISBN 0-19-511346-2. Retrieved 2006-07-19.
- ↑ Tobert JA (2003). "Lovastatin and beyond: the history of the HMG-CoA reductase inhibitors". Nat Rev Drug Discov. 2 (7): 517–26. doi:10.1038/nrd1112. PMID 12815379.
- ↑ "NHS overspends on statins". Jan 2008.
- ↑ "Simvastatin 40mg tablets".
- ↑ Berenson, Alex (23 June 2006). "Merck Loses Protection for Patent on Zocor". New York Times. Retrieved 14 January 2015.
- ↑ "Twenty most prescribed drugs in the community in England in 2013". QualityWatch. Nuffield Trust & Health Foundation. Retrieved 14 January 2015.
External links
- Simvastatin RxList.com
- U.S. National Library of Medicine: Drug Information Portal - Simvastatin
- "Zocor Prescribing information". Merck & Co.