Chondroitin sulfate
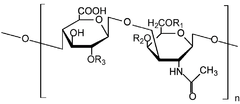
Chondroitin sulfate is a sulfated glycosaminoglycan (GAG) composed of a chain of alternating sugars (N-acetylgalactosamine and glucuronic acid). It is usually found attached to proteins as part of a proteoglycan. A chondroitin chain can have over 100 individual sugars, each of which can be sulfated in variable positions and quantities. Chondroitin sulfate is an important structural component of cartilage and provides much of its resistance to compression.[1] Along with glucosamine, chondroitin sulfate has become a widely used dietary supplement for treatment of osteoarthritis.
Medical use
Although chondroitin is used in dietary supplements as an alternative medicine to treat osteoarthritis and also approved and regulated as a symptomatic slow-acting drug for this disease (SYSADOA) in Europe and some other countries, it is technically neither a medicine nor a disease-modifying treatment.[2][3] See Clinical effects below. It is commonly sold together with glucosamine. Chondroitin and glucosamine are also used in veterinary medicine.[4] Formulated with collagen and wound dressing matrix, one product that uses chondroitin sulfate is the veterinary wound gel Chondroprotec, which is applied over scrapes, burns, and lesions and serves to keep the wound moist and promote healing.[5]
Chondroitin, along with commonly used glucosamine, should not be used to treat patients who have symptomatic osteoarthritis of the knee as evidence shows that these treatments fail to provide relief for that condition.[6]
Adverse effects
Clinical studies have not identified any significant side effects or overdoses of chondroitin sulfate, which suggest its long-term safety.[7] The Task Force of the European League Against Rheumatism (EULAR) committee recently granted chondroitin sulfate a level of toxicity of 6 in a 0-100 scale, confirming it is one of the safest drugs for osteoarthritis.[2] Moreover, its safety is supported by an absence of drug-drug interactions (chondroitin sulfate is not metabolized by cytochrome P450),[8] and the lack of safe alternatives for patients multi-medicated for osteoarthritis and other accompanying diseases, e.g. diabetes, hypertension, hyperlipidemia, etc.
Physical and chemical properties
Chondroitin sulfate chains are unbranched polysaccharides of variable length containing two alternating monosaccharides: D-glucuronic acid (GlcA) and N-acetyl-D-galactosamine (GalNAc). Some GlcA residues are epimerized into L-iduronic acid (IdoA); the resulting disaccharide is then referred to as dermatan sulfate.
Protein attachment
Chondroitin sulfate chains are linked to hydroxyl groups on serine residues of certain proteins. Exactly how proteins are selected for attachment of glycosaminoglycans is not understood. Glycosylated serines are often followed by a glycine and have neighboring acidic residues, but this motif does not always predict glycosylation.
Attachment of the GAG chain begins with four monosaccharides in a fixed pattern: Xyl - Gal - Gal - GlcA. Each sugar is attached by a specific enzyme, allowing for multiple levels of control over GAG synthesis. Xylose begins to be attached to proteins in the endoplasmic reticulum, while the rest of the sugars are attached in the Golgi apparatus.[9]
Sulfation
Each monosaccharide may be left unsulfated, sulfated once, or sulfated twice. In the most common scenario, the hydroxyls of the 4 and 6 positions of the N-acetyl-galactosamine are sulfated, with some chains having the 2 position of glucuronic acid. Sulfation is mediated by specific sulfotransferases. Sulfation in these different positions confers specific biological activities to chondroitin GAG chains.
Structural
Chondroitin sulfate is a major component of extracellular matrix, and is important in maintaining the structural integrity of the tissue. This function is typical of the large aggregating proteoglycans: aggrecan, versican, brevican, and neurocan, collectively termed the lecticans.
As part of aggrecan, chondroitin sulfate is a major component of cartilage. The tightly packed and highly charged sulfate groups of chondroitin sulfate generate electrostatic repulsion that provides much of the resistance of cartilage to compression. Loss of chondroitin sulfate from the cartilage is a major cause of osteoarthritis.
Regulatory
Chondroitin sulfate readily interacts with proteins in the extracellular matrix due to its negative charges. These interactions are important for regulating a diverse array of cellular activities. The lecticans are a major part of the brain extracellular matrix, where the chondroitin sugar chains function to stabilize normal brain synapses as part of perineuronal nets. The levels of chondroitin sulfate proteoglycans are vastly increased after injury to the central nervous system where they act to prevent regeneration of damaged nerve endings. Although these functions are not as well characterized as those of heparan sulfate, new roles continue to be discovered for the chondroitin sulfate proteoglycans.
In cortical development, chondroitin sulfate is expressed by the Sub Plate and acts as a stop signal for neurons migrating from the Ventricular Zone. Neurons stopping here may then be programmed for further migration to specific layers in the cortical plate.
Interactions
Chondroitin's functions depend largely on the properties of the overall proteoglycan of which it is a part. These functions can be broadly divided into structural and regulatory roles. However, this division is not absolute, and some proteoglycans have both structural and regulatory roles (see versican).
Mechanisms of action
The effect of chondroitin sulfate in patients with osteoarthritis is likely the result of a number of reactions including its anti-inflammatory activity, the stimulation of the synthesis of proteoglycans and hyaluronic acid, and the decrease in catabolic activity of chondrocytes inhibiting the synthesis of proteolytic enzymes, nitric oxide, and other substances that contribute to damage cartilage matrix and cause death of articular chondrocytes. A recent review summarizes data from relevant reports describing the biochemical basis of the effect of chondroitin sulfate on osteoarthritis articular tissues.[10] The rationale behind the use of chondroitin sulfate is based on the belief that osteoarthritis is associated with a local deficiency or degradation of natural substances, including internal chondroitin sulfate.
Recently, new mechanisms of action have been described for chondroitin sulfate. In an in vitro study, chondroitin sulfate reduced the IL-1β-induced nuclear factor-kB (NF-κB) translocation in chondrocytes.[11] In addition, chondroitin sulfate has recently shown a positive effect on osteoarthritic structural changes occurred in the subchondral bone.[12]
A review published in the Annals of Rheumatic Diseases describes all the documented evidence regarding the mechanisms of action of chondroitin sulfate[13]
Bioavailability and pharmacokinetics
Pharmacokinetic studies performed on humans and experimental animals after oral administration of chondroitin sulfate revealed that it can be absorbed orally. Chondroitin sulfate shows first-order kinetics up to single doses of 3,000 mg.[14][15][16][17] Multiple doses of 800 mg in patients with osteoarthritis do not alter the kinetics of chondroitin sulfate. The bioavailability of chondroitin sulfate ranges from 15% to 24% of the orally administered dose. More particularly, on the articular tissue, Ronca et al.[18] reported that chondroitin sulfate is not rapidly absorbed in the gastro-intestinal tract and a high content of labeled chondroitin sulfate is found in the synovial fluid and cartilage.
Clinical effects
Various clinical trials performed with chondroitin sulfate have evaluated its efficacy for the treatment of osteoarthritis compared to placebo or non-steroidal anti-inflammatory drugs (NSAIDs). These studies have returned mixed results. Some of the studies have evidenced a significant benefit in terms of pain reduction and improvement of functional capacity, as well as a reduction of joint swelling / effusion, all of them characteristic symptoms of this disease. Specifically, the available data evidence that, even though the clinical effect of the product takes about 2 to 3 weeks to appear, it can reach a global efficacy comparable to that of NSAIDs.[19] Additionally, its effect has been proven to persist for 3 months after treatment suppression (carry-over effect).[20] In the synovial membrane, chondroitin sulfate can significantly reduce joint swelling and effusion;[3] which represents an important benefit considering that the prevalence of synovitis in osteoarthritis has been estimated to be around 50% or higher.[21] Synovitis has also evidenced to aggravate osteoarthritis and accelerate disease progression.[22] Other studies have shown no clinically significant benefit compared to placebo. In 2007, Reichenbach et al.[23][24] used explicit methods to conduct and report[25] a systematic review of 20 trials and concluded "large-scale, methodologically sound trials indicate that the symptomatic benefit of chondroitin is minimal or nonexistent. Use of chondroitin in routine clinical practice should therefore be discouraged." Most of the clinical trials published with chondroitin sulfate have analysed its symptomatic effect on knee osteoarthritis[19][26][27][28][29][30] (Uebelhart D et al., 2004),[31] (Clegg DO et al., 2006)[32] and (Möller I, et al. 2010)[33] However, other studies have also provided evidence of efficacy in finger joints (Verbruggen G et al., 1998);[34] (Verbruggen G et al., 2002),[35] (Gabay C et al., 2011)[36]
Additionally, some clinical trials have also assessed the disease modifying effect of chondroitin sulfate (Uebelhart D et al. 1998);[37] (Verbruggen G. et al., 1998),[34] (Uebelhart D, et al. 2004),[31] (Michel B, et al. 2005),[38] (Verbruggen G, et al. 2002);[35] (Kahan A et al., 2009),[39] (Wildi L, et al. 2011)[40]
The most relevant recently conducted clinical trials performed with chondroitin sulfate are summarized below.
- Clinical trials performed to assess the symptomatic effect of chondroitin sulfate in osteoarthritis:
The largest trial conducted with the product is the Glucosamine and Chondroitin Arthritis Intervention Trial (GAIT), a double-blind, randomized, multicenter clinical trial sponsored by the US National Institutes of Health in 1583 patients with knee osteoarthritis, which was published in the New England Journal of Medicine (Clegg DO, et al. 2006).[32] Patients were randomly assigned to one of five orally administered treatments: two 250 mg capsules of glucosamine hydrochloride three times daily, two 200 mg capsules of chondroitin sulphate three times daily, two capsules of 250 mg of glucosamine hydrochloride plus 200 mg of chondroitin sulphate three times daily, 200 mg of celecoxib daily, or placebo. Treatment was administered for 24 weeks.
- Primary Outcome:
The primary outcome measure was a 20% decrease in the WOMAC pain subscale from baseline to week 24. The analysis of the primary outcome data for all patients showed the percentage of responders in each group to be: glucosamine: 64.0%; chondroitin sulphate: 65.4%; glucosamine + chondroitin sulphate: 66.6%; and celecoxib: 70.1% (p=0.008). Thus, despite the considerable effect elicited by all the products, only the celecoxib group reached statistical significance. This has been discussed by the authors and attributed mainly to the unprecedented high response in the placebo group (60.1%) and the relatively mild degree of pain among participants, which may have limited the ability to detect treatment benefits. Indeed, treatment effects were more substantial in patients with moderate-to-severe pain. Analysis of the results for the primary outcome, based on higher baseline pain, showed the percentage of responders in each treatment group to be 54.3% for placebo; 61.4% for chondroitin sulphate; 65.7% for glucosamine hydrochloride; 69.4% for celecoxib; and 79.2% for combined glucosamine and chondroitin sulphate. Thus, in the higher WOMAC pain stratum, only the combination glucosamine + chondroitin group presented significant efficacy (p=0.002).
- Secondary Outcomes:
Secondary efficacy outcome measures were as follows: OMERACT-OARSI response, 50% decrease in WOMAC pain score, WOMAC pain, stiffness and function score, normalized WOMAC score, patient and investigator global evaluations of disease status and response to study medication, evaluation of the index knee for swelling and tenderness, Health Assessment Questionnaire (HAQ) Alternative Disability score and HAQ Pain score, clinical evaluation for adverse reactions and reconciliation of study medications and rescue analgesia use. Analysis of the OMERACT-OARSI measure in all randomized patients revealed statistical significance for both celecoxib (p=0.007) and glucosamine and chondroitin in combination (P=0.02) compared to placebo. Swelling of study joints was significantly less in patients in the chondroitin sulphate (P=0.01) and celecoxib (P=0.03) treatment arms. Differences in the other secondary outcomes were not statistically significant when compared across the 5 treatment arms for all patients. For patients with moderate-to-severe pain at baseline (n=354), a statistically significant difference was observed in favor of the glucosamine and chondroitin combination group versus placebo for the following efficacy outcomes: 20% decrease in WOMAC pain score (0.002); OMERACT-OARSI response (0.001); 50% decrease in WOMAC pain score (0.02); WOMAC pain score (0.009); WOMAC function score (0.008); normalised WOMAC score (0.017); Health Assessment Questionnaire Pain score (0.03).
A subanalysis of GAIT results based on interaction of Kellgren & Lawrence Grade and Chondroitin Sulfate response relative to placebo was performed by the same study investigators.[41] The results indicated better response for chondroitin sulfate relative to placebo for Kellgren & Lawrence grade 2 compared with grade 3. In general, chondroitin sulfate response within the Kellgren & Lawrence grade 2 group was very similar to that seen for celecoxib. These results suggest that chondroitin sulfate may improve osteoarthritis knee pain in patients with relatively early radiographic disease.
Sawitzke A, et al. 2010[42] evaluated the efficacy and safety of glucosamine and chondroitin sulfate, alone or in combination, as well as celecoxib and placebo on painful knee osteoarthritis over 2 years as a continuation of the GAIT trial. This was a 24-month, double-blind, placebo-controlled study, enrolling 662 patients with knee osteoarthritis who satisfied radiographic criteria (Kellgren/Lawrence grade 2 or 3 changes and baseline joint space width of at least 2 mm). This subset continued to receive their randomized treatment (glucosamine 500 mg three times daily, chondroitin sulfate 400 mg three times daily, the combination of glucosamine and chondroitin sulfate, celecoxib 200 mg daily, or placebo) over 24 months. The primary outcome was a 20% reduction in pain over 24 months as measured by the Western Ontario and McMaster University Osteoarthritis Index (WOMAC). Secondary outcomes included an Outcome Measures in Rheumatology/Osteoarthritis Research Society International response and change from baseline in WOMAC pain and function. Over 2 years, none of the treatments (not even the positive control celecoxib) achieved a clinically important difference in WOMAC pain or function as compared with placebo. Adverse reactions were similar among treatment groups and serious adverse events were rare for all treatments.
History
Chondroitin sulfate was originally isolated well before the structure was characterised, leading to changes in terminology with time.[43] Early researchers identified different fractions of the substance with letters.
| Letter identification | Site of sulfation | Systematic name |
| Chondroitin sulfate A | carbon 4 of the N-acetylgalactosamine (GalNAc) sugar | chondroitin-4-sulfate |
| Chondroitin sulfate C | carbon 6 of the GalNAc sugar | chondroitin-6-sulfate |
| Chondroitin sulfate D | carbon 2 of the glucuronic acid and 6 of the GalNAc sugar | chondroitin-2,6-sulfate |
| Chondroitin sulfate E | carbons 4 and 6 of the GalNAc sugar | chondroitin-4,6-sulfate |
"Chondroitin sulfate B" is an old name for dermatan sulfate, and it is no longer classified as a form of chondroitin sulfate.[44]
Chondroitin, without the "sulfate", has been used to describe a fraction with little or no sulfation.[45] However, this distinction is not used by all.
Although the name "chondroitin sulfate" suggests a salt with a sulfate counter-anion, this is not the case, as sulfate is covalently bonded to the sugar. Rather, since the molecule has multiple negative charges at physiological pH, a cation is present in salts of chondroitin sulfate. Commercial preparations of chondroitin sulfate typically are the sodium salt. Barnhill et al. have suggested that all such preparations of chondroitin sulfate be referred to as "sodium chondroitin" regardless of their sulfation status.[46]
Society and culture
Manufacture
Most chondroitin appears to be made from extracts of cartilaginous cow and pig tissues (cow trachea and pig ear and nose), but other sources such as shark, fish, and bird cartilage are also used. Since chondroitin is not a uniform substance, and is naturally present in a wide variety of forms, the precise composition of each supplement will vary.[46] In fact, although many food supplement companies produce their products in compliance with human food processing Good Manufacturing Practice (GMP), most of them do not produce their products in compliance with the GMP regulations for pharmaceuticals, resulting in products without pharmaceutical requirements.[47] Companies have attempted to produce chondroitin from other substances but have not yet had success.
Recent testing has revealed several flaws in the older testing methods. Without knowing the source of the chondroitin (e.g., shark, porcine, or bovine) and the approximate age of the animal, it is impossible to get a reliable reference standard, and, thus, results from previous testing had yielded percentages between 50 and 400%. In 2007, David Ji et al. reported in the Journal of Analytical Chemistry an extremely accurate method of quantification. The method included using an enzyme to break the chondroitin into its individual unsaturated disaccharides, and then measuring them using HPLC with an ion-pairing column and UV detection.[48]
Legal status
While it is a prescription or over-the-counter drug in 22 countries, chondroitin is regulated in the U.S. as a dietary supplement[49] by the Food and Drug Administration. In Europe, chondroitin sulfate formulations are approved as drugs with evidenced efficacy and safety demonstrated by clinical trials in osteoarthritic patients.[50] Adebowale et al. reported in 2000 that of 32 chondroitin supplements they analysed, only 5 were labeled correctly, and more than half contained less than 40% of the labeled amount.[51] With the introduction of GMP regulations for dietary supplements in 2008, chondroitin sulfate preparations are subject in the US to mandatory labeling standards as well as testing requirements for identity, purity, strength, and composition. United States Pharmacopoeia (USP) testing standards for the identification and quantification of chondroitin are well-established.
There are no FDA regulations on chondroitin sulfate as a food additive, as it is recognized by the FDA as a component of food and is "generally recognized as safe".[52] However, a proposed application of chondroitin sulfate dietary supplement as a means of preventing joint degeneration was highly scrutinized by the FDA, who stated:
" For conventional foods, this evaluation involves considering whether the ingredient that is the source of the substance is generally recognized as safe (GRAS), approved as a food additive, or authorized by a prior sanction issued by FDA (see 21 CFR 101.70(f)). Dietary ingredients in dietary supplements, however, are not subject to the food additive provisions of the act (see section 201(s)(6) of the Act (21 U.S.C. § 321(s)(6)). Rather, they are subject to the adulteration provisions in section 402 of the Act (21 U.S.C. 342) and, if applicable, the new dietary ingredient provisions in section 413 of the Act (21 U.S.C. 350b), which pertain to dietary ingredients that were not marketed in the United States before October 15, 1994."— Letter Regarding the Relationship Between the Consumption of Glucosamine and/or Chondroitin Sulfate and a Reduced Risk of: Osteoarthritis; Osteoarthritis-related Joint Pain, Joint Tenderness, and Joint Swelling; Joint Degeneration; and Cartilage Deterioration
In the same letter, the FDA found that studies performed on the dietary supplement form of chondroitin sulfate were insufficient to substantiate claims that it is efficacious in the prevention of joint deterioration, and denied the request to be allowed to label the supplement as such. They further denied the request to market it as safe, given that no human clinical trials were done, citing that animal studies are not sufficient for the approval of a dietary supplement.[52]
Clinical trials for osteoarthritis
In 2004, a petition was submitted to the FDA that a dietary supplement of chondroitin sulfate be labeled as reducing the risk of osteoarthritis, cartilage deterioration, and osteoarthritis-related joint pain, tenderness, and swelling. The FDA denied the request, stating that experiments conducted by the company did not sufficiently demonstrate the effectiveness of the claim. Among other comments, the FDA noted the poor experimental design of some trials.[52]
In 2007, Reichenbach et al. used explicit methods to conduct and report a systematic review of 20 trials and concluded "large-scale, methodologically sound trials indicate that the symptomatic benefit of chondroitin is minimal or nonexistent. Use of chondroitin in routine clinical practice should therefore be discouraged." In contrast, and also in 2007, Bruyere et al. concluded that "there is compelling evidence that glucosamine sulfate and chondroitin sulfate may interfere with progression of OA."
Contamination in heparin
On Wednesday, March 19, 2008 the U.S. Food and Drug Administration (FDA) identified "oversulfated chondroitin sulfate" as a contaminant in heparin originating from China.[53]
In this regard, "it is very important to remark on the relevant chemical differences between the chondroitin sulfate formulation approved in Europe as a drug and considered the reference product, and the "oversulfated chondroitin sulfate" identified as a contaminant in heparin originating from China."
The "oversulfated chondroitin sulfate" is not a product extracted from biological sources; it is synthesized through a sulfation reaction from the biological molecule. This is a semi-synthesis process that uses naturally derived chondroitin sulfate as a reagent in combination with various potentially dangerous chemicals, though their significance in the toxicity of "oversulfated chondroitin sulfate" is not known.
The resulting product contains 3 or 4 sulfate groups per disaccharide, and, therefore, its structure differs considerably from the original one (see #Sulfation section above). Furthermore, analysis of the contaminant unexpectedly revealed an unusual type of sulfation not found in any natural sources of chondroitin sulfate. In addition, a tetrasulfated disaccharide repeat unit has not been isolated to date from animal tissues[54]
Thus, chondroitin sulfate is merely the substrate of the reaction: The final, oversulfated molecule constitutes a new entity, whose pharmacological and clinical properties are most likely very different from the biological molecule, as it is demonstrated in a recent article published in New England Journal of Medicine. In this study,[55] Sasisekharan and colleagues showed that the oversulfated chondroitin sulfate (OSCS) found in contaminated lots of unfractionated heparin, as well as a synthetically generated OSCS reference standard, directly activated the kinin–kallikrein pathway in human plasma, which can lead to the generation of bradykinin, a potent vasoactive mediator. In addition, OSCS induced generation of C3a and C5a, potent anaphylatoxins derived from complement proteins. Chondroitin sulfate A was also tested and it showed no induction of amidollytic activity. Screening of plasma samples from various species indicated that swine and humans are sensitive to the effects of OSCS in a similar manner. OSCS-containing heparin and synthetically derived OSCS-induced hypotension associated with kallikrein activation when administered by intravenous infusion in swine. In contrast, none of the three pigs treated with chondroitin sulfate A showed any significant changes in blood pressure or heart rate.
See also
- Gout
- Heparin sulfate - a glycosaminoglycan of major pharmaceutical importance for many decades
- Heparan sulfate - a glycosaminoglycan component of proteoglycans in a wide range of vertebrate & invertebrate life
- Methylsulfonylmethane
References
- ↑ Baeurle SA, Kiselev MG, Makarova ES, Nogovitsin EA (2009). "Effect of the counterion behavior on the frictional–compressive properties of chondroitin sulfate solutions". Polymer. 50 (7): 1805–1813. doi:10.1016/j.polymer.2009.01.066.
- 1 2 Jordan KM; Recommendations Arden NK. EULAR (2003). "an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT)". Ann Rheum Dis. 62 (12): 1145–1155. doi:10.1136/ard.2003.011742. PMC 1754382
 . PMID 14644851.
. PMID 14644851. - 1 2 Clegg, Daniel O.; Reda, Domenic J.; Harris, Crystal L.; Klein, Marguerite A.; O'Dell, James R.; Hooper, Michele M.; Bradley, John D.; Bingham, Clifton O.; Weisman, Michael H.; Jackson, Christopher G.; Lane, Nancy E.; Cush, John J.; Moreland, Larry W.; Schumacher, H. Ralph; Oddis, Chester V.; Wolfe, Frederick; Molitor, Jerry A.; Yocum, David E.; Schnitzer, Thomas J.; Furst, Daniel E.; Sawitzke, Allen D.; Shi, Helen; Brandt, Kenneth D.; Moskowitz, Roland W.; Williams, H. James (23 February 2006). "Glucosamine, Chondroitin Sulfate, and the Two in Combination for Painful Knee Osteoarthritis". New England Journal of Medicine. 354 (8): 795–808. doi:10.1056/NEJMoa052771. PMID 16495392. Retrieved 1 March 2015.
- ↑ Forsyth R, Brigden C, Northrop A (2006). "Double blind investigation of the effects of oral supplementation of combined glucosamine hydrochloride (GHCL) and chondroitin sulfate (CS) on stride characteristics of veteran horses". Equine veterinary journal. Supplement. 38 (36): 622–5. doi:10.1111/j.2042-3306.2006.tb05615.x. PMID 17402494.
- ↑ Hydrolyzed Collagen with 10% Chondroitin Sulfate Wound Gel. 510(k) Number: K081724 <http://www.accessdata.fda.gov/cdrh_docs/pdf8/k081724.pdf>
- ↑ American Academy of Orthopaedic Surgeons (February 2013), "Five Things Physicians and Patients Should Question", Choosing Wisely: an initiative of the ABIM Foundation, American Academy of Orthopaedic Surgeons, retrieved 19 May 2013, which cites
- Jevsevar, DS; Brown, GA; Jones, DL; Matzkin, EG; Manner, PA; Mooar, P; Schousboe, JT; Stovitz, S; Sanders, JO; Bozic, KJ; Goldberg, MJ; Martin WR, 3rd; Cummins, DS; Donnelly, P; Woznica, A; Gross, L; American Academy of Orthopaedic Surgeons (Oct 16, 2013). "The American Academy of Orthopaedic Surgeons evidence-based guideline on: treatment of osteoarthritis of the knee, 2nd edition.". The Journal of bone and joint surgery. American volume. 95 (20): 1885–6. PMID 24288804.
- Clegg, Daniel O.; Reda, Domenic J.; Harris, Crystal L.; Klein, Marguerite A.; O'Dell, James R.; Hooper, Michele M.; Bradley, John D.; Bingham, Clifton O.; Weisman, Michael H.; Jackson, Christopher G.; Lane, Nancy E.; Cush, John J.; Moreland, Larry W.; Schumacher, H. Ralph; Oddis, Chester V.; Wolfe, Frederick; Molitor, Jerry A.; Yocum, David E.; Schnitzer, Thomas J.; Furst, Daniel E.; Sawitzke, Allen D.; Shi, Helen; Brandt, Kenneth D.; Moskowitz, Roland W.; Williams, H. James (23 February 2006). "Glucosamine, Chondroitin Sulfate, and the Two in Combination for Painful Knee Osteoarthritis". New England Journal of Medicine. 354 (8): 795–808. doi:10.1056/NEJMoa052771. PMID 16495392.
- Richmond, J; Hunter, D; Irrgang, J; Jones, MH; Levy, B; Marx, R; Snyder-Mackler, L; Watters WC, 3rd; Haralson RH, 3rd; Turkelson, CM; Wies, JL; Boyer, KM; Anderson, S; St Andre, J; Sluka, P; McGowan, R; American Academy of Orthopaedic Surgeons (Sep 2009). "Treatment of osteoarthritis of the knee (nonarthroplasty).". The Journal of the American Academy of Orthopaedic Surgeons. 17 (9): 591–600. PMC 3170838
 . PMID 19726743.
. PMID 19726743.
- ↑ 13. Hathcock JN, Shao a. Risk assessment for glucosamine and chondroitin sulfate. Regulatory Toxicology and Pharmacology, 2007; 47: 78-83
- ↑ Andermann G, Dietz M. The influence of the route of administration on the bioavailability of an endogenous macromolecule: chondroitin sulfate (CSA). Eur J Drug Metab Pharmacokinet 1982;7:11–6
- ↑ Silbert JE, Sugumaran G (2002). "Biosynthesis of chondroitin/dermatan sulfate". IUBMB Life. 54 (4): 177–86. doi:10.1080/15216540214923. PMID 12512856.
- ↑ Monfort J, Pelletier J-P, Garcia-Giralt N, Martel-Pelletier J. Biochemical basis of the effect of chondroitin sulfate on osteoarthritis articular tissues. Ann Rheum Dis 2007; doi:10.1136/ard.2006.068882 .
- ↑ Jomphe C, Gabriac M, Hale TM, Heroux L, Trudeau LE, Deblois D, Montell E, Verges J, du Souich P. Chondroitin Sulfate Inhibits the Nuclear Translocation of Nuclear Factor-kappaB in Interleukin-1beta-Stimulated Chondrocytes. Basic Clin Pharmacol Toxicol. 2007 Nov 5
- ↑ Kwan Tat, S; Pelletier, JP; Verges, J; Lajeunesse, D; Montell, E; Fahmi, H; Lavigne, M; Martel-Pelletier, J (Nov 2007). "Chondroitin and glucosamine sulfate in combination decrease the pro-resorptive properties of human osteoarthritis subchondral bone osteoblasts: a basic science study". Arthritis Research & Therapy. 9 (6): R117. doi:10.1186/ar2325.
- ↑ Monfort J, Pelletier J-P, Garcia-Giralt N, Martel-Pelletier J. Biochemical basis of the effect of chondroitin sulfate on osteoarthritis articular tissues. Ann Rheum Dis, 2008 Jun; 67(6):735-40.
- ↑ Conte A, Palmieri L, Segnini D, Ronca G. Metabolic fate of partially depolymerized chondroitin sulfate administered to the rat. Drugs Exp Clin Res. 1991;17:27-33.
- ↑ Conte A, de Bernardi M, Palmieri L, Lualdi P, Mautone G, Ronca G. Metabolic fate of exogenous chondroitin sulfate in man" Arzneimittelforschung 1991;41:768-72.
- ↑ Conte A, Volpi N, Palmieri L, Bahous I, Ronca G. Biochemical and pharmacokinetic aspects of oral treatment with chondroitin sulfate" Arzneimittelforschung 1995;45:918-25.
- ↑ Palmieri L, Conte A, Giovannini L, Lualdi P, Ronca G. Metabolic fate of exogenous chondroitin sulfate in the experimental animal" Arzneimittelforschung 1990;40:319-23.
- ↑ Ronca F, Palmieri L, Panicucci P, Ronca G. Anti-inflammatory activity of chondroitin sulfate. Osteoarthritis Cartilage. 1998;6 Suppl A:14-21.
- 1 2 Morreale, P; Manopulo, R; Galati, M; Boccanera, L; Saponati, G; Bocchi, L (August 1996). "Comparison of the antiinflammatory efficacy of chondroitin sulfate and diclofenac sodium in patients with knee osteoarthritis.". The Journal of rheumatology. 23 (8): 1385–91. PMID 8856618.
- ↑ Uebelhart, D; Malaise, M; Marcolongo, R; de Vathaire, F; Piperno, M; Mailleux, E; Fioravanti, A; Matoso, L; Vignon, E (April 2004). "Intermittent treatment of knee osteoarthritis with oral chondroitin sulfate: a one-year, randomized, double-blind, multicenter study versus placebo.". Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 12 (4): 269–76. doi:10.1016/j.joca.2004.01.004. PMID 15023378.
- ↑ Loeuille, Damien; Chary-Valckenaere, Isabelle; Champigneulle, Jacqueline; Rat, Anne-Christine; Toussaint, Frédéric; Pinzano-Watrin, Astrid; Goebel, Jean Christophe; Mainard, Didier; Blum, Alain; Pourel, Jacques; Netter, Patrick; Gillet, Pierre (November 2005). "Macroscopic and microscopic features of synovial membrane inflammation in the osteoarthritic knee: Correlating magnetic resonance imaging findings with disease severity". Arthritis & Rheumatism. 52 (11): 3492–3501. doi:10.1002/art.21373. Retrieved 1 March 2015.
- ↑ Ayral, X; Pickering, E; Woodworth, T; Mackillop, N; Dougados, M (May 2005). "Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis – results of a 1 year longitudinal arthroscopic study in 422 patients". Osteoarthritis and Cartilage. 13 (5): 361–367. doi:10.1016/j.joca.2005.01.005. Retrieved 1 March 2015.
- ↑ Reichenbach S, Sterchi R, Scherer M, Trelle S, Bürgi E, Bürgi U, Dieppe PA, Jüni P (2007). "Meta-analysis: chondroitin for osteoarthritis of the knee or hip". Ann Intern Med. 146 (8): 580–90. doi:10.7326/0003-4819-146-8-200704170-00009. PMID 17438317.
- ↑ Lane N (2007). "Review: based on evidence from higher-quality trials, chondroitin does not reduce pain in knee or hip osteoarthritis". ACP J. Club. 147 (2): 44. PMID 17764135.
- ↑ Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF (1999). "Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses". Lancet. 354 (9193): 1896–900. doi:10.1016/S0140-6736(99)04149-5. PMID 10584742.
- ↑ Bourgeois, P; Chales, G; Dehais, J; Delcambre, B; Kuntz, JL; Rozenberg, S (May 1998). "Efficacy and tolerability of chondroitin sulfate 1200 mg/day vs chondroitin sulfate 3 x 400 mg/day vs placebo.". Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 6 Suppl A: 25–30. doi:10.1016/s1063-4584(98)80008-3. PMID 9743816.
- ↑ Bucsi, L; Poór, G (May 1998). "Efficacy and tolerability of oral chondroitin sulfate as a symptomatic slow-acting drug for osteoarthritis (SYSADOA) in the treatment of knee osteoarthritis.". Osteoarthritis and Cartilage / OARS, Osteoarthritis Research Society. 6 Suppl A: 31–6. doi:10.1016/s1063-4584(98)80009-5. PMID 9743817.
- ↑ Pavelka K, Bucsi L, Manopulo R. Double-blind, dose effect study of oral CS 4&6 1200 mg, 800 mg, 200 mg against placebo in the treatment of femoro-tibial osteoarthritis. Litera Rheumatol. 1998, 24: 21-30.
- ↑ Uebelhart, D; Thonar, EJ; Delmas, PD; Chantraine, A; Vignon, E (May 1998). "Effects of oral chondroitin sulfate on the progression of knee osteoarthritis: a pilot study.". Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 6 Suppl A: 39–46. doi:10.1016/s1063-4584(98)80011-3. PMID 9743819.
- ↑ Mazieres, B; Combe, B; Phan Van, A; Tondut, J; Grynfeltt, M (January 2001). "Chondroitin sulfate in osteoarthritis of the knee: a prospective, double blind, placebo controlled multicenter clinical study.". The Journal of rheumatology. 28 (1): 173–81. PMID 11196521. Retrieved 1 March 2015.
- 1 2 Uebelhart D, et al. Intermittent treatment of knee osteoarthritis with oral chondroitin sulfate: a one-year, randomized, double-blind, multicenter study versus placebo Osteoarthritis Cartilage. 2004 Apr;12(4):269-76.
- 1 2 Clegg, DO; et al. (2006). "Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis". N Engl J Med. 354 (8): 795–808. doi:10.1056/nejmoa052771.
- ↑ Möller, I; et al. (Jun 2010). "Effectiveness of chondroitin sulphate in patients with concomitant knee osteoarthritis and psoriasis: a randomized, double-blind, placebo-controlled study". Osteoarthritis Cartilage. 18 (Suppl 1): S32–40. doi:10.1016/j.joca.2010.01.018.
- 1 2 Verbruggen G, et al. Chondroitin sulfate: S/DMOAD in the treatment of finger joint OA. Osteoarthritis Cart 1998, 6 (Suppl. A): 37-38.
- 1 2 Verbruggen, G; et al. (2002). "Systems to assess the effects of progression of finger joint OA and the effects of S/DMOAD". Clinical Rheumatology. 21 (3): 231–243. doi:10.1007/s10067-002-8290-7.
- ↑ Gabay, C; et al. (Nov 2011). "Symptomatic effects of chondroitin 4 and chondroitin 6 sulfate on hand osteoarthritis: a randomized, double-blind, placebo-controlled clinical trial at a single center". Arthritis Rheum. 63 (11): 3383–91. doi:10.1002/art.30574.
- ↑ Uebelhart D, Thonar EJMA, Delmas PD, Chantraine A, Vignon E. Effects of oral chondroitin sulfate on the progression of knee osteoarthritis: a pilot study. Osteoarth Cart 1998;6 Supp A: 39-46.
- ↑ Michel, BA; et al. (Mar 2005). "Chondroitins 4 and 6 sulfate in osteoarthritis of the knee: a randomized, controlled trial". Arthritis Rheum. 52 (3): 779–86. doi:10.1002/art.20867.
- ↑ Kahan, A; et al. (Feb 2009). "Long-Term Effects of Chondroitins 4 and 6 Sulfate on Knee Osteoarthritis". Arthritis Rheum. 58 (11): 524–533. doi:10.1002/art.24255.
- ↑ Wildi LM, et al. Chondroitin sulphate reduces both cartilage volume loss and bone marrow lesions in knee osteoarthritis patients starting as early as 6 months after initiation of therapy: a randomised, double-blind, placebo-controlled pilot study using MRI. Ann Rheum Dis. 2011 Jun;70(6):982-9.
- ↑ Clegg, Daniel O.; Reda, Domenica J.; Shi, Helen (2005). "Chondroitin sulfate may have differential effects on oa symptoms related to degree of radiographic involvement". Osteoarthritis Cartilage. 13 (Supplement 1): S76–S77. doi:10.1016/S1063-4584(05)80490-X. Retrieved 1 March 2015.
- ↑ Sawitzke, Allen D.; Shi, Helen; Finco, Martha F.; Dunlop, Dorothy D.; Harris, Crystal L.; Singer, Nora G.; Bradley, John D.; Silver, David; Jackson, Christopher G.; Lane, Nancy E.; Oddis, Chester V.; Wolfe, Fred; Lisse, Jeffrey; Furst, Daniel E.; Bingham, Clifton O.; Reda, Domenic J.; Moskowitz, Roland W.; Williams, H. James; Clegg, Daniel O. (4 June 2010). "Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT". Annals of the Rheumatic Diseases. 69 (8): 1459–1464. doi:10.1136/ard.2009.120469. PMC 3086604
 . PMID 20525840. Retrieved 1 March 2015.
. PMID 20525840. Retrieved 1 March 2015. - ↑ P. A. Levene; F. B. La Forge (1913). "On Chondroitin Sulphuric Acid". J. Biol. Chem. 15: 69–79. Free PDF online
- ↑ Chondroitin sulfates at the US National Library of Medicine Medical Subject Headings (MeSH)
- ↑ Davidson EA, Meyer K (1954). "Chondroitin, a new mucopolysaccharide". J Biol Chem. 211 (2): 605–11. PMID 13221568. Free PDF online
- 1 2 Barnhill JG, Fye CL, Williams DW, Reda DJ, Harris CL, Clegg DO (2006). "Chondroitin product selection for the glucosamine/chondroitin arthritis intervention trial". J Am Pharm Assoc (Wash DC). 46 (1): 14–24. doi:10.1331/154434506775268616. PMID 16529337.
- ↑ Jamie G. Barnhill, Carol L. Fye, David W. Williams, Domenic J. Reda, Crystal L. Harris, and Daniel O. Clegg. Chondroitin Product Selection for the Glucosamine/Chondroitin Arthritis Intervention Trial. J Am Pharm Assoc. 2006; 46:14–24.
- ↑ Ji D, Roman M, Zhou J, Hildreth J (2007). "Determination of Chondroitin Sulfate Content in Raw Materials and Dietary Supplements by High-Performance Liquid Chromatography with Ultraviolet Detection After Enzymatic Hydrolysis: Single-Laboratory Validation". J AOAC Int. 90 (3): 659–669. PMC 2614115
 . PMID 17580617.
. PMID 17580617. - ↑ "Questions and Answers: NIH Glucosamine/Chondroitin Arthritis Intervention Trial Primary Study", Backgrounder, National Center for Complementary and Integrative Medicine, retrieved 2013-10-17
- ↑ Vergés J, Castañeda-Hernández, G. On the bioavailability of oral chondroitin sulfate formulations: proposed criteria for bioequivalence studies. Proc. West. Pharmacol. Soc., 2004; 47: 50-53
- ↑ Adebowale AO Cox DS, Liang Z, Eddington ND (2000). "Analysis of glucosamine and chondroitin sulfate content in marketed products and the Caco-2 permeability of chondroitin sulfate raw materials". J Am Nutr Assoc. 3: 37–44. Archived from the original on April 7, 2006.
- 1 2 3 Letter Regarding the Relationship Between the Consumption of Glucosamine and/or Chondroitin Sulfate and a Reduced Risk of: Osteoarthritis; Osteoarthritis-related Joint Pain, Joint Tenderness, and Joint Swelling; Joint Degeneration; and Cartilage Deterioration(Docket No. 2004P-0059). Addressed to John W. Emford, Esq. William K. Hubbard. Oct. 7, 2004. <http://www.fda.gov/food/ingredientspackaginglabeling/labelingnutrition/ucm073400.htm>
- ↑ Zawisza, Julie (2008-03-19). "FDA Media Briefing on Heparin" (PDF). U.S. Food and Drug Administration. Retrieved 2008-04-23.
- ↑ Guerrini, M.; Beccati, D.; Shriver, Z.; Naggi, A.; Viswanathan, K.; Bisio, A.; Capila, I.; Lansing, C.; Guglieri, S.; Fraser, B.; Al-Hakim, A.; Gunay, N. S.; Zhang, Z.; Robinson, L.; Buhse, L.; Nasr, M.; Woodcock, J.; Langer, R.; Venkataraman, G.; Linhardt, R. J.; Casu, B.; Torri, G.; Sasisekharan, R. (Jun 2008). "Oversulfated chondroitin sulfate is a contaminant in heparin associated with adverse clinical events". Nature Biotechnology. 26 (6): 669–675. doi:10.1038/nbt1407. ISSN 1087-0156. PMC 3491566
 . PMID 18437154.
. PMID 18437154. - ↑ Kishimoto, K.; Viswanathan, K.; Ganguly, T.; Elankumaran, S.; Smith, S.; Pelzer, K.; Lansing, C.; Sriranganathan, N.; Zhao, G.; Galcheva-Gargova, Z.; Al-Hakim, A.; Bailey, G. S.; Fraser, B.; Roy, S.; Rogers-Cotrone, T.; Buhse, L.; Whary, M.; Fox, J.; Nasr, M.; Dal Pan, G. J.; Shriver, Z.; Langer, R. S.; Venkataraman, G.; Austen, K. F.; Woodcock, J.; Sasisekharan, R. (Jun 2008). "Contaminated heparin associated with adverse clinical events and activation of the contact system". The New England Journal of Medicine. 358 (23): 2457–2467. doi:10.1056/NEJMoa0803200. ISSN 0028-4793. PMC 3778681
 . PMID 18434646.
. PMID 18434646.
External links
- General Glucosamine and Chondroitin Sulfate information at Arthritis Foundation
- "Product Review: Joint Supplements (Glucosamine, Chondroitin, and MSM)" Summary of a Consumer Labs test of the actual composition of these supplements at consumerlabs.com
- "Glucosamine/Chondroitin Products Not Measuring Up", News report of the analysis of commercial supplements by Adebowale et al. at WebMD
- "Chondroitin Sulfate Manufacturing and Risk of Mad Cow Disease" by Winston Wicomb, Ph.D., September 24, 2002. Information on methods for extraction of chondroitin sulfate from cow trachea, at the Stone Clinic of San Francisco, at stoneclinic.com
- "Testing Status: Chondroitin Sulfate M030009", A thorough review of available information on the use of chondroitin sulfate in humans from the National Toxicology Program at National Institute of Environmental Health Sciences
- Chondroitin Sulfate, summary of information on the use of chondroitin sulfate from the publishers of the Physicians' Desk Reference.
- "Glucosamine/Chondroitin Arthritis Intervention Trial (GAIT)," ClinicalTrials.gov information on the purpose, design, and analysis of the study at clinicaltrials.gov
- "NIH News: Efficacy of Glucosamine and Chondroitin Sulfate May Depend on Level of Osteoarthritis Pain", Wednesday, February 22, 2006 at National Institutes of Health