Tenidap
 | |
| Clinical data | |
|---|---|
| ATC code | M01AX23 (WHO) |
| Identifiers | |
| |
| PubChem (CID) | 60712 |
| ChemSpider |
54717 |
| UNII |
9K7CJ74ONH |
| KEGG |
D06072 |
| ChEMBL |
CHEMBL1097558 |
| Chemical and physical data | |
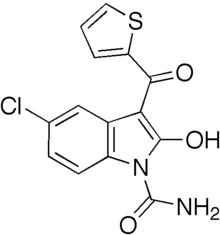
| Formula | C14H9ClN2O3S |
| Molar mass | 320.75086 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Tenidap was a COX/5-LOX inhibitor and cytokine-modulating anti-inflammatory drug candidate[1] that was under development by Pfizer as a promising potential treatment for rheumatoid arthritis,[2] but Pfizer halted development after marketing approval was rejected by the FDA in 1996[3] due to liver and kidney toxicity, which was attributed to metabolites of the drug with a thiophene moiety that caused oxidative damage.[4]
References
- ↑ Wylie, G.; Appelboom, T.; Bolten, W.; Breedveld, F. C.; Feely, J.; Leeming, M. R.; Le Loët, X.; Manthorpe, R.; Marcolongo, R.; Smolen, J. (1995). "A comparative study of tenidap, a cytokine-modulating anti-rheumatic drug, and diclofenac in rheumatoid arthritis: A 24-week analysis of a 1-year clinical trial". British journal of rheumatology. 34 (6): 554–563. doi:10.1093/rheumatology/34.6.554. PMID 7543348.
- ↑ Staff, American Journal of Nursing. Drug Watch: Tenidap Offers Arthritis Therapy Minus Toxicity AJN 1996 96(1):58
- ↑ Pfizer. Sept 27, 1996 Press release: Pfizer To Halt Plans For Commercialization Of Tenidap For Rheumatoid Arthritis
- ↑ Hwang SH et al. Rationally designed multitarget agents against inflammation and pain. Curr Med Chem. 2013;20(13):1783-99. PMID 23410172 PMC 4113248
This article is issued from Wikipedia - version of the 10/15/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.