2-succinyl-6-hydroxy-2,4-cyclohexadiene-1-carboxylate synthase
| 2-succinyl-6-hydroxy-2,4-cyclohexadiene-1-carboxylate synthase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 4.2.99.20 | ||||||||
| CAS number | 122007-88-9 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
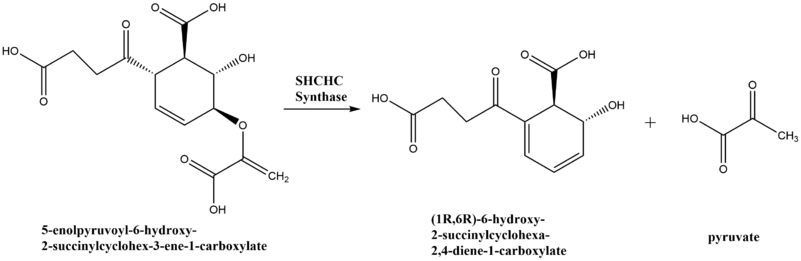
2-succinyl-6-hydroxy-2,4-cyclohexadiene-1-carboxylate synthase, also known as SHCHC synthase, is encoded by the menH gene in E.coli and functions in the synthesis of vitamin K.[1] The specific step in the synthetic pathway that SHCHC synthase catalyzes is the conversion of 5-enolpyruvoyl-6-hydroxy-2-succinylcyclohex-3-ene-1-carboxylate to (1R,6R)-6-hydroxy-2-succinylcyclohexa-2,4-diene-1-carboxylate and pyruvate.[2]
Background
Vitamin K is a fat soluble vitamin known to aid in blood clotting. It is recommended that all newborns receive an injection of vitamin K in order to prevent excessive bleeding of the brain after birth. There are two major forms of vitamin K that occur naturally. Phylloquinone, also known as K1, is synthesized by plants and is the major form of vitamin K in the diet. Menaquinone, K2, includes a range of forms that are synthesized by bacteria in the gut.[3]
Vitamin K is synthesized from the molecule chorismate in a nine step conversion process. SHCHC synthase catalyzes the third step in the process.[4]
Chemistry
Reaction scheme
Enzyme Structure
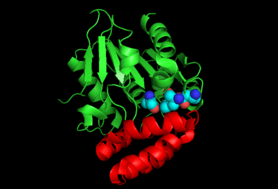
The crystal structure of the MenH enzyme in E.coli (SHCHC synthase) exists as a complex of three protein molecules shown in the diagram. SHCHC synthase forms an alpha/beta hydrolase fold with a central set of seven parallel beta sheets surrounded by alpha helixes on both sides. A cap of five alpha helixes serves to enclose the active site.[6] The enzyme exists in an open form until it binds the substrate, when it morphs into a closed form with an active catalytic triad.[7]
Energetic analysis shows that SHCHC synthase has a low energetic burden for catalytic activity.[1] This means the enzyme is more prone to mutation and is one of the most diverse enzymes in the vitamin K synthetic pathway.[8] Only fifteen amino acid residues are absolutely conserved across mutations of the enzyme.[8]
Catalytic Mechanism
The active site contains a catalytic triad of syrine, histine and arginine, which is conserved across all mutants and is proposed to initiate the reaction.[1] The triad residues are located at Ser86, Asp210, and His232.[6] This triad is proposed to catalyze a proton extraction which triggers a transfer of electrons leading to the elimination of pyruvate and formation of SHCHC.[7] Originally, it was proposed that the transition state was stabilized by a nontraditional oxyanion hole. Now a traditional oxyanion hole is favored, but not definitive.[6]
Reaction Mechanism
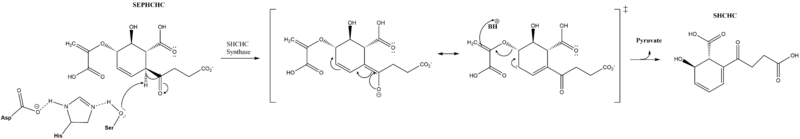
Cofactors and Alternate Reactions
SHCHC synthase is unaffected by traditional cofactors such as divalent metal ions and EDTA.[1] The enzyme is fairly specific and only acts on SEPHCHC and close derivatives.[2]
Controversy
MenH (SHCHC synthase) was previously thought to be a thioesterase involved in hydrolyzing DHNA-CoA in a later step of menaquinone synthesis. In 2008, it was determined that MenH has poor catalytic activity toward palmitoyl-CoA, casting doubt on its role as a thioesterase.[1] Direct analysis confirmed that MenH is unable to hydrolyze DHNA-CoA.[1] In 2009, it was proposed that a dedicated hotdog fold thioesterase would be needed to catalyze the hydrolysis of DHNA-CoA.[9] A protein was identified in 2013 that could fit this role.[10]
References
- 1 2 3 4 5 6 Jiang; et al. (2008-02-20). "Identification and Characterization of (1R,6R)-2-Succinyl-6-hydroxy-2,4-cyclohexadiene-1-carboxylate Synthase in the Menaquinone Biosynthesis of Escherichia coli". Biochemistry. 47: 3426–3434. doi:10.1021/bi7023755.
- 1 2 "Information on EC 4.2.99.20 - 2-succinyl-6-hydroxy-2,4-cyclohexadiene-1-carboxylate synthase". Brenda: The Comprehensive Enzyme Information System. TU Braunschwieg. 2014-07-01. Retrieved 2014-11-01.
- ↑ "Micronutrient Information Center". Linus Pauling Institute. 2014-11-30. Retrieved 2014-11-30.
- ↑ van Oostende C, Widhalm JR, Furt F, Ducluzeau AL, Basset GJC (2011) Phylloquinone (Vitamin K1): function, enzymes and genes. in Advances in Botanical Research, eds Fabrice Rébeillé and Roland Douce, 59: 229-61, Academic Press (Amsterdam).
- ↑ Johnston; et al. (2013-01-01). "Crystal Structure of E.coli MenH". RCSB Protein Data Bank. doi:10.2210/pdb4gdm/pdb. Retrieved 2014-11-24.
- 1 2 3 Johnston; et al. (2013-04-18). "Crystal Structures of E. coli Native MenH and Two Active Site Mutants". PLOS ONE. 8: e61325. doi:10.1371/journal.pone.0061325.
- 1 2 Sun; et al. (2013-11-22). "Molecular Basis of the General Base Catalysis of an α/β-Hydrolase Catalytic Triad". Journal of Biological Chemistry. 289: 15867–15879. doi:10.1074/jbc.M113.535641.
- 1 2 Jiang; et al. (2009-06-22). "Catalytic Mechanism of SHCHC Synthase in the Menaquinone Biosynthesis of Escherichia coli: Identification and Mutational Analysis of the Active Site Residues". Biochemistry. 48: 6921–6931. doi:10.1021/bi900897h.
- ↑ Widhalm; et al. (2009-04-07). "A dedicated thioesterase of the Hotdog-fold family is required for the biosynthesis of the naphthoquinone ring of vitamin K1". PNAS. 106: 5599–603. doi:10.1073/pnas.0900738106. PMID 19321747.
- ↑ Chen; et al. (2013-06-01). "Identification of a Hotdog Fold Thioesterase Involved in the Biosynthesis of Menaquinone in Escherichia coli". Journal of Bacteriology. 195: 2768–2775. doi:10.1128/JB.00141-13.