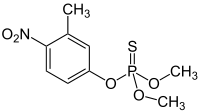
Fenitrothion
 | |
 | |
| Names | |
|---|---|
| IUPAC name
O,O-Dimethyl O-(3-methyl-4-nitrophenyl) phosphorothioate | |
| Other names
• Dimethoxy-(3-methyl-4-nitrophenoxy)thioxophosphorane O,O-Dimethyl O-4-nitro-m-tolyl phosphorothioate | |
| Identifiers | |
| 122-14-5 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:34757 |
| ChEMBL | ChEMBL347698 |
| ChemSpider | 28941 |
| ECHA InfoCard | 100.004.114 |
| KEGG | C14442 |
| PubChem | 31200 |
| UNII | W8M4X3Y7ZY |
| |
| |
| Properties | |
| C9H12NO5PS | |
| Molar mass | 277.23 g·mol−1 |
| Appearance | Yellow-brown liquid |
| Density | 1.3227 g/cm3 |
| Melting point | 3.4 °C (38.1 °F; 276.5 K) |
| Boiling point | 118 °C (244 °F; 391 K) at 0.05 mmHg |
| 38.0 mg/L | |
| Solubility | Readily soluble in dichloromethane, 2-propanol, toluene, hardly soluble in n-hexane.[1] |
| log P | 3.30 (octanol/water)[2] |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
Rat, oral: 500 mg/kg[3] Mouse (female), oral: 1416 mg/kg[4] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Fenitrothion (IUPAC name: O,O-Dimethyl O-(3-methyl-4-nitrophenyl) phosphorothioate) is a phosphorothioate (organophosphate) insecticide; cheap and widely used worldwide.
In experiments fenitrothion at sublethal doses affected the motor movement of marsupials,[5] and at acute dose levels it reduced the energy of birds.[6]
In chronic (low) dose tests, unexpectedly only the lowest concentration (0.011 microgram/liter) of fenitrothion depressed the growth of an algae, though all of the chronic dose levels used were toxic in other ways to the algae.[7]
Just half of fenitrothion's minimally effective dose altered the thyroid structure of a freshwater murrel (the snakehead fish).[8]
In an unusual demonstration of resistance to pesticides, 8% of insects in farm fields were found to carry a symbiotic gut microbe that can metabolize and detoxify fenitrothion; after in-vitro tests showed that the microbe significantly increased the survival of fenitrothion-treated insects.[9]
References
- ↑ Farm Chemicals Handbook 1999. Willoughby, OH: Meister Publishing Co., 1999., p. C 177
- ↑ Hansch, C., Leo, A., D. Hoekman. Exploring QSAR - Hydrophobic, Electronic, and Steric Constants. Washington, DC: American Chemical Society., 1995., p. 60
- ↑ Kirk-Othmer Encyclopedia of Chemical Technology. 3rd ed., Volumes 1-26. New York, NY: John Wiley and Sons, 1978-1984., p. V13 440 (1981)
- ↑ WHO; Environ Health Criteria 133: Fenitrothion p.70 (1992)
- ↑ Buttemer, William A.; Story, Paul G.; Fildes, Karen J.; Baudinette, Russell V.; Astheimer, Lee B. (2008). "Fenitrothion, an organophosphate, affects running endurance but not aerobic capacity in fat-tailed dunnarts (Sminthopsis crassicaudata)". Chemosphere. 72 (9): 1315–1320. doi:10.1016/j.chemosphere.2008.04.054. PMID 18547601.
- ↑ Kitulagodage, Malsha; Isanhart, John; Buttemer, William A.; Hooper, Michael J.; Astheimer, Lee B. (2011). "Fipronil toxicity in northern bobwhite quail Colinus virginianus: Reduced feeding behaviour and sulfone metabolite formation". Chemosphere. 83 (4): 524–530. doi:10.1016/j.chemosphere.2010.12.057. PMID 21227481.
- ↑ Ferrando, M; Sancho, E; Andreu-Moliner, E (1996). "Chronic Toxicity of Fenitrothion to an Algae (Nannochloris oculata), a Rotifer (Brachionus calyciflorus), and the Cladoceran (Daphnia magna)". Ecotoxicology and Environmental Safety. 35 (2): 112–120. doi:10.1006/eesa.1996.0090. PMID 8950533.
- ↑ Saxena, P; Mani, K (1988). "Effect of safe concentrations of some pesticides on thyroid in the freshwater murrel, Channa punctatus: A histopathological study". Environmental Pollution. 55 (2): 97–105. doi:10.1016/0269-7491(88)90121-2. PMID 15092506.
- ↑ Kikuchi, Y.; Hayatsu, M.; Hosokawa, T.; Nagayama, A.; Tago, K.; Fukatsu, T. (2012). "Symbiont-mediated insecticide resistance". Proceedings of the National Academy of Sciences. 109 (22): 8618. Bibcode:2012PNAS..109.8618K. doi:10.1073/pnas.1200231109.
External links
- Fenitrothion in the Pesticide Properties DataBase (PPDB)
- Compendium of Pesticide Common Names (source of data)
- Hazardous Substances Data Bank (source of data)
- Inchem
- Entox
- Re-evaluation of Fenitrothion by the Pest Management Regulatory Agency of Canada