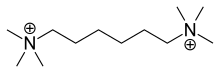
Hexamethonium
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
60-26-4 |
| PubChem (CID) | 3604 |
| IUPHAR/BPS | 3963 |
| DrugBank |
DB08960 |
| ChemSpider |
3478 |
| UNII |
3C9PSP36Z2 |
| ChEMBL |
CHEMBL105608 |
| Chemical and physical data | |
| Formula | C12H30N2 |
| Molar mass | 202.38 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Hexamethonium is a non-depolarising ganglionic blocker, a nicotinic nACh (NN) receptor antagonist[1] that acts in autonomic ganglia by binding mostly in or on the NN receptor, and not the acetylcholine binding site itself. It does not have any effect on the muscarinic acetylcholine receptors (mAChR) located on target organs of the parasympathetic nervous system but acts as antagonist at the nicotinic acetylcholine receptors located in sympathetic and parasympathetic ganglia (NN).[2]
Pharmacology
It can act on receptors at pre-ganglionic sites in both the sympathetic and parasympathetic nervous systems, which are both regulated by nicotinic ligand-gated ionotropic acetylcholine receptors. Postganglionic sympathetic systems are usually regulated by norepinephrine (noradrenaline) (adrenergic receptors), whereas parasympathetic systems are acetylcholine-based, and instead rely on muscarinic receptors (some post-ganglionic sympathetic neurons, such as those stimulating sweat glands, release acetylcholine).
The organ system and adverse effects of ganglion blockers are due to the parasympathetic and sympathetic stimuli blockage at preganglionic sites. Side-effects include combined sympatholytic (e.g., orthostatic hypotension and sexual dysfunction) and parasympatholytic (e.g., constipation, urinary retention, glaucoma, blurry vision, decreased lacrimal gland secretion, dry mouth (xerostomia)) effects.
Uses
It was formerly used to treat disorders, such as chronic hypertension, of the peripheral nervous system, which is innervated only by the sympathetic nervous system. The non-specificity of this treatment led to discontinuing its use.[3]
The use of inhaled hexamethonium, an unapproved drug, in a normal volunteer during a medical study is believed to have caused or contributed to her death[4][5] in light of the presence of abnormal "ground glass opacities" on her chest X-ray.
See also
References
- ↑ "Hexamethonium - Compound Summary". http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=3604. 2013-06-18.
- ↑ Howland RD, Mycek MJ 2006. Lippincott's illustrated reviews: Pharmacology. 3:d edition, page 47.
- ↑ Hardman et al., Goodman and Gilman's The Pharmacological Basis of Therapeutics, 10th edition, 2001, pp 210-211.
- ↑ "Johns Hopkins' Tragedy: Could Librarians Have Prevented a Death?". Retrieved 2008-10-06.
- ↑ Savulescu J, Spriggs M (February 2002). "The hexamethonium asthma study and the death of a normal volunteer in research". Journal of Medical Ethics. 28 (1): 3–4. doi:10.1136/jme.28.1.3. PMC 1733509
 . PMID 11834748.
. PMID 11834748.