Mepyramine
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a606008 |
| ATC code | R06AC01 (WHO) D04AA02 (WHO) |
| Identifiers | |
| |
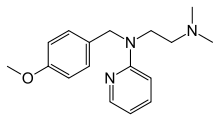
| Synonyms | N-[2-(dimethylamino)ethyl]-N-[(4-methoxyphenyl)methyl]pyridin-2-amine |
| CAS Number |
91-84-9 59-33-6 (maleate) |
| PubChem (CID) | 4992 |
| IUPHAR/BPS | 1227 |
| DrugBank |
DB06691 |
| ChemSpider |
4818 |
| UNII |
HPE317O9TL |
| KEGG |
D08183 |
| ChEBI |
CHEBI:6762 |
| ChEMBL |
CHEMBL511 |
| ECHA InfoCard | 100.001.912 |
| Chemical and physical data | |
| Formula | C17H23N3O |
| Molar mass | 285.38 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Mepyramine, also known as pyrilamine, is a first generation antihistamine, targeting the H1 receptor.[1] However, it rapidly permeates the brain often causing drowsiness. It also has anticholinergic properties. It is used in over-the-counter combination products to treat the common cold and menstrual symptoms.[2] It is also the active ingredient of the topical antihistamine creams Anthisan and Neoantergan, sold for the treatment of insect bites, stings, and nettle rash.
Synthesis
See also
- Chloropyramine (chloro instead of methoxy)
References
- ↑ Parsons, Mike E.; Ganellin, C. Robin (January 2006). "Histamine and its receptors". British Journal of Pharmacology. 147 (S1): S127–S135. doi:10.1038/sj.bjp.0706440. PMC 1760721
 . PMID 16402096.
. PMID 16402096. - ↑ Active Ingredients for Midol Complete
- ↑ R.J. Horclois, U.S. Patent 2,502,151 (1950).
- ↑ Huttrer, C. P.; Djerassi, C.; Beears, W. L.; Mayer, R. L.; Scholz, C. R. (1946). "Heterocyclic amines with antithistaminic activity". Journal of the American Chemical Society. 68 (10): 1999–2002. doi:10.1021/ja01214a037. PMID 21001124.
- ↑ D. Bovet, R. Horclois, F. Walthert, C.R. Soc. Biol., 138, 99 (1944).
| Antihistamines for topical use | |
|---|---|
| Anesthetics for topical use | |
| Others |
|
| Aminoalkyl ethers |
|
|---|---|
| Substituted alkylamines | |
| Substituted ethylenediamines |
|
| Phenothiazine derivatives | |
| Piperazine derivatives | |
| Others for systemic use |
|
| For topical use | |
| Psychedelics (5-HT2A agonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dissociatives (NMDAR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Deliriants (mAChR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Receptor (ligands) |
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transporter (inhibitors) |
| ||||||||||||||
| Enzyme (inhibitors) |
| ||||||||||||||
| Others |
| ||||||||||||||
This article is issued from Wikipedia - version of the 4/2/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.
