HK3
| HK3 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||
| Identifiers | |||||||||||||||
| Aliases | HK3, HKIII, HXK3, hexokinase 3 | ||||||||||||||
| External IDs | MGI: 2670962 HomoloGene: 55633 GeneCards: HK3 | ||||||||||||||
| Orthologs | |||||||||||||||
| Species | Human | Mouse | |||||||||||||
| Entrez | |||||||||||||||
| Ensembl | |||||||||||||||
| UniProt | |||||||||||||||
| RefSeq (mRNA) | |||||||||||||||
| RefSeq (protein) | |||||||||||||||
| Location (UCSC) | Chr 5: 176.88 – 176.9 Mb | Chr 13: 55.01 – 55.02 Mb | |||||||||||||
| PubMed search | [1] | [2] | |||||||||||||
| Wikidata | |||||||||||||||
| View/Edit Human | View/Edit Mouse |
Hexokinase 3 also known as HK3 is an enzyme which in humans is encoded by the HK2 gene on chromosome 5.[3][4] Hexokinases phosphorylate glucose to produce glucose-6-phosphate (G6P), the first step in most glucose metabolism pathways. This gene encodes hexokinase 3. Similar to hexokinases 1 and 2, this allosteric enzyme is inhibited by its product glucose-6-phosphate. [provided by RefSeq, Apr 2009][5]
Structure
HK3 is one of four highly homologous hexokinase isoforms in mammalian cells.[6][7][8][9] This protein has a molecular weight of 100 kDa and is composed of two highly similar 50-kDa domains at its N- and C-terminals.[7][8][9][10][11] This high similarity, along with the and the existence of a 50-kDa hexokinase (HK4), suggests that the 100-kDa hexokinases originated from a 50-kDa precursor via gene duplication and tandem ligation.[8][11] Like with HK1, only the C-terminal domain possesses catalytic ability, whereas the N-terminal domain is predicted to contain glucose and G6P binding sites, as well as a 32-residue region essential for proper protein folding.[7][8] Moreover, the catalytic activity depends on the interaction between the two terminal domains.[8] Unlike HK1 and HK2, HK3 lacks a mitochondrial binding sequence at its N-terminal.[8][12][13]
Function
As a cytoplasmic isoform of hexokinase and a member of the sugar kinase family, HK3 catalyzes the rate-limiting and first obligatory step of glucose metabolism, which is the ATP-dependent phosphorylation of glucose to G6P.[8][9][14] Physiological levels of G6P can regulate this process by inhibiting HK3 as negative feedback, though inorganic phosphate can relieve G6P inhibition.[7][11] Inorganic phosphate can also directly regulate HK3, and the double regulation may better suit its anabolic functions.[7] By phosphorylating glucose, HK3 effectively prevents glucose from leaving the cell and, thus, commits glucose to energy metabolism.[7][8][10][11] Compared to HK1 and HK2, HK3 possesses a higher affinity for glucose and will bind the substrate even at physiological levels, though this binding may be attenuated by intracellular ATP.[7] Uniquely, HK3 can be inhibited by glucose at high concentrations.[12][15] HK3 is also less sensitive to G6P inhibition.[7][12]
Despite its lack of mitochondrial association, HK3 also functions to protect the cell against apoptosis.[8][14] Overexpression of HK3 has resulted in increased ATP levels, decreased reactive oxygen species (ROS) production, attenuated reduction in the mitochondrial membrane potential, and enhanced mitochondrial biogenesis. Overall, HK3 may promote cell survival by controlling ROS levels and boosting energy production. Currently, only hypoxia is known to induce HK3 expression through a HIF-dependent pathway. The inducible expression of HK3 indicates its adaptive role in metabolic responses to changes in the cellular environment.[8]
In particular, HK3 is ubiquitously expressed in tissues, albeit at relatively low abundance.[7][8][11][15] Higher abundance levels have been cited in lung, kidney, and liver tissue.[7][8][12] Within cells, HK3 localizes to the cytoplasm and putatively binds the perinuclear envelope.[8][12][13] HK3 is the predominant hexokinase in myeloid cells, particularly granulocytes.[16]
Clinical Significance
HK3 is found to be overexpressed in malignant follicular thyroid nodules. In conjunction with cyclin A and galectin-3, HK3 could be used as diagnostic biomarker to screen for malignancy in patients.[14][17] Meanwhile, HK3 was found to be repressed in acute myeloid leukemia (AML) blast cells and acute promyelocytic leukemia (APL) patients. The transcription factor PU.1 is known to directly activate transcription of the antiapoptotic BCL2A1 gene or inhibit transcription of the p53 tumor suppressor to promote cell survival, and is proposed to also directly activate HK3 transcription during neutrophil differentiation to support short-term cell survival of mature neutrophils.[13] Regulators repressing HK3 expression in AML include PML-RARA and CEBPA.[13][16] Regarding acute lymphoblastic leukemia (ALL), functional enrichment analysis revealed HK3 as a key gene and suggests that HK3 shares antiapoptotic function with HK1 and HK2.[14]
Interactions
The HK3 promoter is known to interact with PU.1,[13] PML-RARA,[13] and CEBPA.[16]
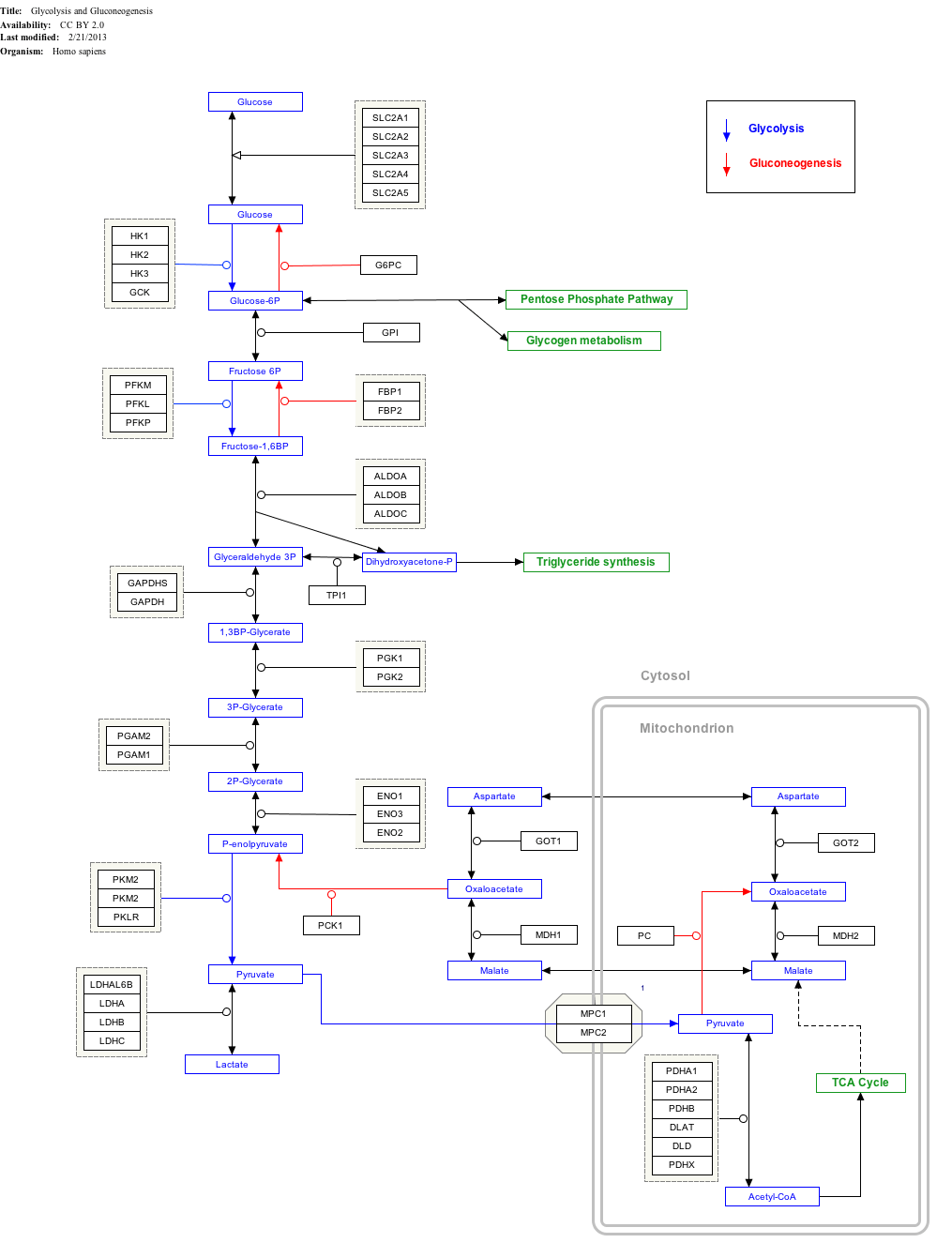
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
Glycolysis and Gluconeogenesis edit
- ↑ The interactive pathway map can be edited at WikiPathways: "GlycolysisGluconeogenesis_WP534".
See also
References
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Furuta H, Nishi S, Le Beau MM, Fernald AA, Yano H, Bell GI (Aug 1996). "Sequence of human hexokinase III cDNA and assignment of the human hexokinase III gene (HK3) to chromosome band 5q35.2 by fluorescence in situ hybridization". Genomics. 36 (1): 206–9. doi:10.1006/geno.1996.0448. PMID 8812439.
- ↑ Colosimo A, Calabrese G, Gennarelli M, Ruzzo AM, Sangiuolo F, Magnani M, Palka G, Novelli G, Dallapiccola B (1996). "Assignment of the hexokinase type 3 gene (HK3) to human chromosome band 5q35.3 by somatic cell hybrids and in situ hybridization". Cytogenetics and Cell Genetics. 74 (3): 187–8. doi:10.1159/000134409. PMID 8941369.
- ↑ "Entrez Gene: HK3 hexokinase 3 (white cell)".
- ↑ Murakami K, Kanno H, Tancabelic J, Fujii H (2002). "Gene expression and biological significance of hexokinase in erythroid cells". Acta Haematologica. 108 (4): 204–9. doi:10.1159/000065656. PMID 12432216.
- 1 2 3 4 5 6 7 8 9 10 Okatsu K, Iemura S, Koyano F, Go E, Kimura M, Natsume T, Tanaka K, Matsuda N (Nov 2012). "Mitochondrial hexokinase HKI is a novel substrate of the Parkin ubiquitin ligase". Biochemical and Biophysical Research Communications. 428 (1): 197–202. doi:10.1016/j.bbrc.2012.10.041. PMID 23068103.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 Wyatt E, Wu R, Rabeh W, Park HW, Ghanefar M, Ardehali H (3 November 2010). "Regulation and cytoprotective role of hexokinase III". PLOS ONE. 5 (11): e13823. doi:10.1371/journal.pone.0013823. PMC 2972215
 . PMID 21072205.
. PMID 21072205. - 1 2 3 Reid S, Masters C (1985). "On the developmental properties and tissue interactions of hexokinase". Mechanisms of Ageing and Development. 31 (2): 197–212. doi:10.1016/s0047-6374(85)80030-0. PMID 4058069.
- 1 2 Aleshin AE, Zeng C, Bourenkov GP, Bartunik HD, Fromm HJ, Honzatko RB (Jan 1998). "The mechanism of regulation of hexokinase: new insights from the crystal structure of recombinant human brain hexokinase complexed with glucose and glucose-6-phosphate". Structure. 6 (1): 39–50. doi:10.1016/s0969-2126(98)00006-9. PMID 9493266.
- 1 2 3 4 5 Printz RL, Osawa H, Ardehali H, Koch S, Granner DK (Feb 1997). "Hexokinase II gene: structure, regulation and promoter organization". Biochemical Society Transactions. 25 (1): 107–12. doi:10.1042/bst0250107. PMID 9056853.
- 1 2 3 4 5 Lowes W, Walker M, Alberti KG, Agius L (Jan 1998). "Hexokinase isoenzymes in normal and cirrhotic human liver: suppression of glucokinase in cirrhosis". Biochimica et Biophysica Acta. 1379 (1): 134–42. doi:10.1016/s0304-4165(97)00092-5. PMID 9468341.
- 1 2 3 4 5 6 Federzoni EA, Valk PJ, Torbett BE, Haferlach T, Löwenberg B, Fey MF, Tschan MP (May 2012). "PU.1 is linking the glycolytic enzyme HK3 in neutrophil differentiation and survival of APL cells". Blood. 119 (21): 4963–70. doi:10.1182/blood-2011-09-378117. PMID 22498738.
- 1 2 3 4 Gao HY, Luo XG, Chen X, Wang JH (Jan 2015). "Identification of key genes affecting disease free survival time of pediatric acute lymphoblastic leukemia based on bioinformatic analysis". Blood Cells, Molecules & Diseases. 54 (1): 38–43. doi:10.1016/j.bcmd.2014.08.002. PMID 25172542.
- 1 2 Cárdenas ML, Cornish-Bowden A, Ureta T (Mar 1998). "Evolution and regulatory role of the hexokinases". Biochimica et Biophysica Acta. 1401 (3): 242–64. doi:10.1016/s0167-4889(97)00150-x. PMID 9540816.
- 1 2 3 Federzoni EA, Humbert M, Torbett BE, Behre G, Fey MF, Tschan MP (3 March 2014). "CEBPA-dependent HK3 and KLF5 expression in primary AML and during AML differentiation". Scientific Reports. 4: 4261. doi:10.1038/srep04261. PMID 24584857.
- ↑ Hooft L, van der Veldt AA, Hoekstra OS, Boers M, Molthoff CF, van Diest PJ (Feb 2008). "Hexokinase III, cyclin A and galectin-3 are overexpressed in malignant follicular thyroid nodules". Clinical Endocrinology. 68 (2): 252–7. doi:10.1111/j.1365-2265.2007.03031.x. PMID 17868400.
Further reading
- Reid S, Masters C (1985). "On the developmental properties and tissue interactions of hexokinase". Mechanisms of Ageing and Development. 31 (2): 197–212. doi:10.1016/S0047-6374(85)80030-0. PMID 4058069.
- Rijksen G, Staal GE, Beks PJ, Streefkerk M, Akkerman JW (Dec 1982). "Compartmentation of hexokinase in human blood cells. Characterization of soluble and particulate enzymes". Biochimica et Biophysica Acta. 719 (3): 431–7. doi:10.1016/0304-4165(82)90230-6. PMID 7150652.
- Adkins JN, Varnum SM, Auberry KJ, Moore RJ, Angell NH, Smith RD, Springer DL, Pounds JG (Dec 2002). "Toward a human blood serum proteome: analysis by multidimensional separation coupled with mass spectrometry". Molecular & Cellular Proteomics. 1 (12): 947–55. doi:10.1074/mcp.M200066-MCP200. PMID 12543931.
- Palma F, Agostini D, Mason P, Dachà M, Piccoli G, Biagiarelli B, Fiorani M, Stocchi V (Feb 1996). "Purification and characterization of the carboxyl-domain of human hexokinase type III expressed as fusion protein". Molecular and Cellular Biochemistry. 155 (1): 23–9. doi:10.1007/BF00714329. PMID 8717435.
- Povey S, Corney G, Harris H (May 1975). "Genetically determined polymorphism of a form of hexokinase, HK III, found in human leucocytes". Annals of Human Genetics. 38 (4): 407–15. doi:10.1111/j.1469-1809.1975.tb00630.x. PMID 1190733.
- Anderson NL, Anderson NG (Nov 2002). "The human plasma proteome: history, character, and diagnostic prospects". Molecular & Cellular Proteomics. 1 (11): 845–67. doi:10.1074/mcp.R200007-MCP200. PMID 12488461.
- He C, Kraft P, Chen C, Buring JE, Paré G, Hankinson SE, Chanock SJ, Ridker PM, Hunter DJ, Chasman DI (Jun 2009). "Genome-wide association studies identify loci associated with age at menarche and age at natural menopause". Nature Genetics. 41 (6): 724–8. doi:10.1038/ng.385. PMC 2888798
 . PMID 19448621.
. PMID 19448621. - Fonteyne P, Casneuf V, Pauwels P, Van Damme N, Peeters M, Dierckx R, Van de Wiele C (Aug 2009). "Expression of hexokinases and glucose transporters in treated and untreated oesophageal adenocarcinoma". Histology and Histopathology. 24 (8): 971–7. PMID 19554504.
- Sui D, Wilson JE (Oct 2000). "Interaction of insulin-like growth factor binding protein-4, Miz-1, leptin, lipocalin-type prostaglandin D synthase, and granulin precursor with the N-terminal half of type III hexokinase". Archives of Biochemistry and Biophysics. 382 (2): 262–74. doi:10.1006/abbi.2000.2019. PMID 11068878.
- Lowes W, Walker M, Alberti KG, Agius L (Jan 1998). "Hexokinase isoenzymes in normal and cirrhotic human liver: suppression of glucokinase in cirrhosis". Biochimica et Biophysica Acta. 1379 (1): 134–42. doi:10.1016/s0304-4165(97)00092-5. PMID 9468341.
- Furuta H, Nishi S, Le Beau MM, Fernald AA, Yano H, Bell GI (Aug 1996). "Sequence of human hexokinase III cDNA and assignment of the human hexokinase III gene (HK3) to chromosome band 5q35.2 by fluorescence in situ hybridization". Genomics. 36 (1): 206–9. doi:10.1006/geno.1996.0448. PMID 8812439.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.