HSAB theory
The HSAB concept is an initialism for "hard and soft (Lewis) acids and bases". Also known as the Pearson acid base concept, HSAB is widely used in chemistry for explaining stability of compounds, reaction mechanisms and pathways. It assigns the terms 'hard' or 'soft', and 'acid' or 'base' to chemical species. 'Hard' applies to species which are small, have high charge states (the charge criterion applies mainly to acids, to a lesser extent to bases), and are weakly polarizable. 'Soft' applies to species which are big, have low charge states and are strongly polarizable.[1] The concept is a way of applying the notion of orbital overlap to specific chemical cases.
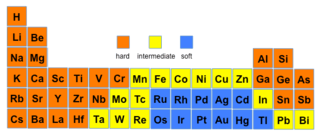
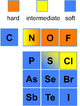
The theory is used in contexts where a qualitative, rather than quantitative, description would help in understanding the predominant factors which drive chemical properties and reactions. This is especially so in transition metal chemistry, where numerous experiments have been done to determine the relative ordering of ligands and transition metal ions in terms of their hardness and softness.
HSAB theory is also useful in predicting the products of metathesis reactions. In 2005 it was shown that even the sensitivity and performance of explosive materials can be explained on basis of HSAB theory.[2]
Ralph Pearson introduced the HSAB principle in the early 1960s[3][4][5] as an attempt to unify inorganic and organic reaction chemistry.[6]
Theory
| Hard-soft trends for acids and bases | ||||
|---|---|---|---|---|
|
The gist of this theory is that soft acids react faster and form stronger bonds with soft bases, whereas hard acids react faster and form stronger bonds with hard bases, all other factors being equal.[7] The classification in the original work was mostly based on equilibrium constants for reaction of two Lewis bases competing for a Lewis acid.
| Property | Hard acids and bases | Soft acids and bases |
|---|---|---|
| atomic/ionic radius | small | large |
| oxidation state | high | low or zero |
| polarizability | low | high |
| electronegativity (bases) | high | low |
| HOMO energy of bases[7][8] | low | higher |
| LUMO energy of acids[7][8] | high | lower (but > soft-base HOMO) |
| affinity | ionic bonding | covalent bonding |
| Acids | Bases | ||||||
|---|---|---|---|---|---|---|---|
| hard | soft | hard | soft | ||||
| Hydronium | H3O+ | Mercury | CH3Hg+, Hg2+, Hg22+ | Hydroxide | OH− | Hydride | H− |
| Alkali metals | Li+,Na+,K+ | Platinum | Pt2+ | Alkoxide | RO− | Thiolate | RS− |
| Titanium | Ti4+ | Palladium | Pd2+ | Halogens | F−,Cl− | Halogens | I− |
| Chromium | Cr3+,Cr6+ | Silver | Ag+ | Ammonia | NH3 | Phosphine | PR3 |
| Boron trifluoride | BF3 | borane | BH3 | Carboxylate | CH3COO− | Thiocyanate | SCN− |
| Carbocation | R3C+ | P-chloranil | Carbonate | CO32− | Carbon monoxide | CO | |
| Lanthanides | Ln3+ | bulk Metals | M0 | Hydrazine | N2H4 | Benzene | C6H6 |
| Thorium | Th4+ | Gold | Au+ | ||||
Borderline cases are also identified: borderline acids are trimethylborane, sulfur dioxide and ferrous Fe2+, cobalt Co2+ caesium Cs+ and lead Pb2+ cations. Borderline bases are: aniline, pyridine, nitrogen N2 and the azide, chloride, bromide, nitrate and sulfate anions.
Generally speaking, acids and bases interact and the most stable interactions are hard-hard (ionogenic character) and soft-soft (covalent character).
An attempt to quantify the 'softness' of a base consists in determining the equilibrium constant for the following equilibrium:
- BH + CH3Hg+ ⇌ H+ + CH3HgB
Where CH3Hg+ (methylmercury ion) is a very soft acid and H+ (proton) is a hard acid, which compete for B (the base to be classified).
Some examples illustrating the effectiveness of the theory:
- Bulk metals are soft acids and are poisoned by soft bases such as phosphines and sulfides.
- Hard solvents such as hydrogen fluoride, water and the protic solvents tend to solvate strong solute bases such as the fluorine anion and the oxygen anions. On the other hand, dipolar aprotic solvents such as dimethyl sulfoxide and acetone are soft solvents with a preference for solvating large anions and soft bases.
- In coordination chemistry soft-soft and hard-hard interactions exist between ligands and metal centers.
Chemical hardness
| Acids | Bases | ||||
|---|---|---|---|---|---|
| Hydrogen | H+ | ∞ | Fluoride | F− | 7 |
| Aluminium | Al3+ | 45.8 | Ammonia | NH3 | 6.8 |
| Lithium | Li+ | 35.1 | hydride | H− | 6.8 |
| Scandium | Sc3+ | 24.6 | carbon monoxide | CO | 6.0 |
| Sodium | Na+ | 21.1 | hydroxyl | OH− | 5.6 |
| Lanthanum | La3+ | 15.4 | cyanide | CN− | 5.3 |
| Zinc | Zn2+ | 10.8 | phosphane | PH3 | 5.0 |
| Carbon dioxide | CO2 | 10.8 | nitrite | NO2− | 4.5 |
| Sulfur dioxide | SO2 | 5.6 | Hydrosulfide | SH− | 4.1 |
| Iodine | I2 | 3.4 | Methane | CH3− | 4.0 |
| | |||||
In 1983 Pearson together with Robert Parr extended the qualitative HSAB theory with a quantitative definition of the chemical hardness (η) as being proportional to the second derivative of the total energy of a chemical system with respect to changes in the number of electrons at a fixed nuclear environment:[9]
- .
The factor of one-half is arbitrary and often dropped as Pearson has noted.[10]
An operational definition for the chemical hardness is obtained by applying a three-point finite difference approximation to the second derivative:[11]
where I is the ionization potential and A the electron affinity. This expression implies that the chemical hardness is proportional to the band gap of a chemical system, when a gap exists.
The first derivative of the energy with respect to the number of electrons is equal to the chemical potential, μ, of the system,
- ,
from which an operational definition for the chemical potential is obtained from a finite difference approximation to the first order derivative as
which is equal to the negative of the electronegativity (χ) definition on the Mulliken scale: μ = −χ.
The hardness and Mulliken electronegativity are related as
- ,
and in this sense hardness is a measure for resistance to deformation or change. Likewise a value of zero denotes maximum softness, where softness is defined as the reciprocal of hardness.
In a compilation of hardness values only that of the hydride anion deviates. Another discrepancy noted in the original 1983 article are the apparent higher hardness of Tl3+ compared to Tl+.
Modifications
If the interaction between acid and base in solution results in an equilibrium mixture the strength of the interaction can be quantified in terms of an equilibrium constant. An alternative quantitative measure is the standard heat (enthalpy) of formation of the adduct in a non-coordinating solvent. Drago and Wayland proposed a two-parameter equation which predicts the formation of a very large number of adducts quite accurately.
Value of the E and C parameters can be found in Drago et al.[12] Hancock and Martell found that an E and C equation analogous to that of Drago gave an excellent quantitative prediction of formation constants for complexes of 34 metal ions plus the proton with a wide range of unidentate Lewis acids in aqueous solution, and also offered insights into factors governing HSAB behavior in solution.[13]
Another quantitative system has been proposed, in which Lewis acid strength is based on gas-phase affinity for fluoride. [14]
Kornblum's rule
An application of HSAB theory is the so-called Kornblum's rule which states that in reactions with ambident nucleophiles (nucleophiles that can attack from two or more places), the more electronegative atom reacts when the reaction mechanism is SN1 and the less electronegative one in a SN2 reaction. This rule (established in 1954) [15] actually predates HSAB theory but in HSAB terms its explanation is that in a SN1 reaction the carbocation (a hard acid) reacts with a hard base (high electronegativity) and that in a SN2 reaction tetravalent carbon (a soft acid) reacts with soft bases.
According to recent findings electrophilic alkylations at free CN− occur preferentially at carbon, regardless of whether the SN1 or SN2 mechanism is involved and whether hard or soft electrophiles are employed. Preferred N attack, as postulated for hard electrophiles by the HSAB principle, could not be observed with any alkylating agent. Isocyano compounds are only formed with highly reactive electrophiles that react without an activation barrier because the diffusion limit is approached. It is claimed that the knowledge of absolute rate constants and not of the hardness of the reaction partners is needed to predict the outcome of alkylations of the cyanide ion.[16]
Criticism
In 2011, Herbert Mayr et al. from Ludwig Maximilian University of Munich (LMU) published a critical review in Angewandte Chemie.[17] Consecutive analysis of various types of ambident organic system reveals that an older approach based on thermodynamic/kinetic control describes reactivity of organic compounds perfectly, while the HSAB principle actually fails and should be abandoned in the rationalization of ambident reactivity of organic compounds.
See also
References
- ↑ Jolly, W. L. (1984). Modern Inorganic Chemistry. New York: McGraw-Hill. ISBN 0-07-032760-2.
- ↑ E.-C. Koch, Acid-Base Interactions in Energetic Materials: I. The Hard and Soft Acids and Bases (HSAB) Principle-Insights to Reactivity and Sensitivity of Energetic Materials, Prop.,Expl.,Pyrotech. 30 2005, 5
- ↑ Pearson, Ralph G. (1963). "Hard and Soft Acids and Bases". J. Am. Chem. Soc. 85 (22): 3533–3539. doi:10.1021/ja00905a001.
- ↑ Pearson, Ralph G. (1968). "Hard and soft acids and bases, HSAB, part 1: Fundamental principles". J. Chem. Educ. 1968 (45): 581–586. Bibcode:1968JChEd..45..581P. doi:10.1021/ed045p581. (subscription required (help)).
- ↑ Pearson, Ralph G. (1968). "Hard and soft acids and bases, HSAB, part II: Underlying theories". J. Chem. Educ. 1968 (45): 643–648. Bibcode:1968JChEd..45..643P. doi:10.1021/ed045p643. (subscription required (help)).
- ↑ R. G. Pearson, Chemical Hardness - Applications From Molecules to Solids, Wiley-VCH, Weinheim, 1997, 198 pp
- 1 2 3 IUPAC, Glossary of terms used in theoretical organic chemistry, accessed 16 Dec 2006.
- 1 2 Miessler G.L. and Tarr D.A. "Inorganic Chemistry" 2nd ed. Prentice-Hall 1999, p.181-5
- 1 2 Robert G. Parr & Ralph G. Pearson (1983). "Absolute hardness: companion parameter to absolute electronegativity". J. Am. Chem. Soc. 105 (26): 7512–7516. doi:10.1021/ja00364a005.
- ↑ Ralph G. Pearson (2005). "Chemical hardness and density functional theory" (PDF). J. Chem. Sci. 117 (5): 369–377. doi:10.1007/BF02708340.
- ↑ Delchev, Ya. I.; A. I. Kuleff; J. Maruani; Tz. Mineva; F. Zahariev (2006). Jean-Pierre Julien; Jean Maruani; Didier Mayou, eds. Strutinsky's shell-correction method in the extended Kohn-Sham scheme: application to the ionization potential, electron affinity, electronegativity and chemical hardness of atoms in Recent Advances in the Theory of Chemical and Physical Systems. New York: Springer-Verlag. pp. 159–177. ISBN 978-1-4020-4527-1.
- ↑ Drago, Russell S.; Wong, Ngai; Bilgrien, Carl; Vogel, Glenn C. (1987). "E and C parameters from Hammett substituent constants and use of E and C to understand cobalt-carbon bond energies". Inorganic Chemistry. 26: 9–14. doi:10.1021/ic00248a003.
- ↑ Hancock, R. D.; Martell, A. E. (1989). "Ligand design for the selective complexation of metal ions in aqueous solution.". Chemical Reviews. 89: 1875–1914. doi:10.1021/cr00098a011.
- ↑ Christe, K.O.; Dixon, D.A.; McLemore, D.; Wilson, W.W.; Sheehy, J.A.; Boatz, J.A. (2000). "On a quantitative scale for Lewis acidity and recent progress in polynitrogen chemistry". Journal of Fluorine Chemistry. 101 (2): 151–153. doi:10.1016/S0022-1139(99)00151-7. ISSN 0022-1139.
- ↑ The Mechanism of the Reaction of Silver Nitrite with Alkyl Halides. The Contrasting Reactions of Silver and Alkali Metal Salts with Alkyl Halides. The Alkylation of Ambident Anions Nathan Kornblum, Robert A. Smiley, Robert K. Blackwood, Don C. Iffland J. Am. Chem. Soc.; 1955; 77(23); 6269-6280. doi:10.1021/ja01628a064
- ↑ Tishkov, Alexander A.; Mayr, Herbert (2004). "Ambident Reactivity of the Cyanide Ion: A Failure of the HSAB Principle". Angewandte Chemie International Edition. 44 (1): 142–145. doi:10.1002/anie.200461640.
- ↑ http://onlinelibrary.wiley.com/doi/10.1002/anie.201007100/abstract Angew. Chem. Int. Ed. 2011, 50, 6470 – 6505, "Farewell to the HSAB Treatment of Ambident Reactivity"