MAX phases
The MAX phases are layered, hexagonal carbides and nitrides have the general formula: Mn+1AXn, (MAX) where n = 1 to 3, M is an early transition metal, A is an A-group (mostly IIIA and IVA, or groups 13 and 14) element and X is either carbon and/or nitrogen. The layered structure consists of edge-sharing, distorted XM6 octahedra interleaved by single planar layers of the A-group element.
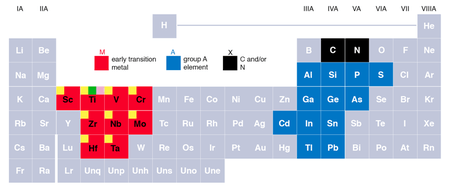
| 211 Phases | 312 Phases | 413 Phases |
|---|---|---|
| Ti2CdC, Sc2InC, Ti2AlC, Ti2GaC, Ti2InC, Ti2TlC, V2AlC, V2GaC, Cr2GaC, Ti2AlN, Ti2GaN, Ti2InN, V2GaN, Cr2GaN, Ti2GeC, Ti2SnC, Ti2PbC, V2GeC, Cr2AlC, Cr2GeC, V2PC, V2AsC, Ti2SC, Zr2InC, Zr2TlC, Nb2AlC, Nb2GaC, Nb2InC, Mo2GaC, Zr2InN, Zr2TlN, Zr2SnC, Zr2PbC, Nb2SnC, Nb2PC, Nb2AsC, Zr2SC, Nb2SC, Hf2InC, Hf2TlC, Ta2AlC, Ta2GaC, Hf2SnC, Hf2PbC, Hf2SnN, Hf2SC | Ti3AlC2, V3AlC2, |
Ti4AlN3, V4AlC3, |
History
In the 1990s, the ternary compound, Ti3SiC2, was synthesized and fully characterized for the first time by Dr. Michel Barsoum's research group at Drexel University. A year later they showed that this compound was but one of over sixty phases,[2] most discovered and produced in powder form in the 1960s by H. Nowotny and coworkers.[3] In 1999 they discovered Ti4AlN3 and realized that they were dealing with a much larger family of solids that all behaved similarly. Since 1996, when the first paper was published on the subject,[4] tremendous progress has been made in understanding the properties of these phases.
Synthesis
The synthesis of the ternary compound, Ti3SiC2 has been realized by different methods, including combustion synthesis, chemical vapor deposition, physical vapor deposition, arc melting, hot isostatic pressing, SHS, reactive sintering, spark plasma sintering, and mechanical alloying.[5][6][7][8]
Properties
These carbides and nitrides possess unusual combination of chemical, physical, electrical, and mechanical properties, exhibiting both metallic and ceramic characteristics under various conditions.[9][10] These include high electrical and thermal conductivity, thermal shock resistance, damage tolerance, machinability, high elastic stiffness, and low thermal expansion coefficients. Some MAX phases are also highly resistant to chemical attack (e.g. Ti3SiC2) and high-temperature oxidation in air (Ti2AlC, Cr2AlC, and Ti3AlC2). They are useful in technologies involving high efficiency engines, damage tolerant thermal systems, increasing fatigue resistance, and retention of rigidity at high temperatures.[11] These properties can be related to the electronic structure of MAX phases. It can be described as periodic alteration of high and low electron density regions.[12] This allows for design of other nanolaminates based on the electronic structure similarities, such as Mo2BC[13] and PdFe3N.[14]
Electrical
The MAX phases are electrically and thermally conductive due to their metallic-like nature of bonding. Most of the MAX phases are better electric and thermal conductors than Ti. This is also related to the electronic structure.[15]
Physical
While MAX phases are stiff, they can be machined as easily as some metals. They can all be machined using a manual hacksaw, despite the fact that some of them are three times as stiff as titanium metal, with the same density as titanium. They can also be polished to a metallic luster because of their excellent electrical conductivities. They are not susceptible to thermal shock and exceptionally damage tolerant. Some, such as Ti2AlC and Cr2AlC, are oxidation and corrosion resistant.[16] Polycrystalline Ti3SiC2 has zero thermopower that is correlated to the anisotropic electronic structure.[17]
Mechanical
The MAX phases as a class are generally stiff, lightweight, and plastic at high temperatures. Due to the layered atomic structure of these compounds, some, like Ti3SiC2 and Ti2AlC, are also creep and fatigue resistant,[18] and maintain their strengths to high temperatures. They exhibit unique deformation characterized by basal slip (evidences of out-of-basal plane dislocations and dislocation cross-slips were recently reported in MAX phase deformed at high temperature[19]), a combination of kink and shear band deformation, and delaminations of individual grains.[20][21][22] During mechanical testing, it has been found that polycrystalline Ti3SiC2 cylinders can be repeatedly compressed at room temperature, up to stresses of 1 GPa, and fully recover upon the removal of the load while dissipating 25% of the energy. It was by characterizing these unique mechanical properties of the MAX phases that kinking non-linear solids were discovered. The micromechanism supposed to be responsible for these properties is the incipient kink band (IKB). However no direct evidence of these IKBs has been yet obtained, thus leaving the door open to other mechanism that are less assumption-hungry. Indeed, a recent study demonstrates that the reversible hysteretic loops when cycling MAX polycrystals can be as well explained by the complex response of the very anisotropic lamellar microstructure.[23]
Potential applications
- Tough, machinable, thermal shock-resistant refractories[24]
- High-temperature heating elements[25]
- Coatings for electrical contacts
- Neutron irradiation resistant parts for nuclear applications [26]
- Precursor for the synthesis of carbide-derived carbon [27]
- Precursor for the synthesis of MXenes, a family of two-dimensional transition metal carbides, nitrides, and carbonitrides [28]
References
- ↑ Eklund, P., Beckers, M., Jansson, U., Högberg, H., & Hultman, L. (2010). "The Mn+1AXn phases: Materials science and thin-film processing". Thin Solid Films. 518 (8): 1851–1878. doi:10.1016/j.tsf.2009.07.184.
- ↑ Barsoum, M.W., Brodkin, D., & El-Raghy, T. (1997). "Layered Machinable Ceramics For High Temperature Applications". Scrip. Met. Et. Mater. 36: 535–541. doi:10.1016/s1359-6462(96)00418-6.
- ↑ Nowotny, H. (1970). "Struktuchemie Einiger Verbindungen der Ubergangsmetalle mit den elementen C, Si, Ge, Sn". Prog. Solid State Chem. 2: 27. doi:10.1016/0079-6786(71)90016-1.
- ↑ Barsoum, M.W. & El-Raghy, T. (1996). "Synthesis and Characterization of a Remarkable Ceramic: Ti3SiC2". J. Amer. Cer. Soc. 79 (7): 1953–1956. doi:10.1111/j.1151-2916.1996.tb08018.x.
- ↑ Yin, Xi; Chen, Kexin; Zhou, Heping; Ning, Xiaoshan (August 2010). "Combustion Synthesis of Ti3SiC2/TiC Composites from Elemental Powders under High-Gravity Conditions". Journal of the American Ceramic Society. 93 (8): 2182–2187. doi:10.1111/j.1551-2916.2010.03714.x.
- ↑ Arunajatesan, Sowmya; Carim, Altaf H. (March 1995). "Synthesis of Titanium Silicon Carbide". Journal of the American Ceramic Society. 78 (3): 667–672. doi:10.1111/j.1151-2916.1995.tb08230.x.
- ↑ Gao, N. F.; Miyamoto, Y.; Zhang, D. (1999). "Dense Ti3SiC2 prepared by reactive HIP". Journal of Materials Science. 34 (18): 4385–4392. doi:10.1023/A:1004664500254.
- ↑ Li, Shi-Bo; Zhai, Hong-Xiang (8 June 2005). "Synthesis and Reaction Mechanism of Ti3SiC2 by Mechanical Alloying of Elemental Ti, Si, and C Powders". Journal of the American Ceramic Society. 88 (8): 2092–2098. doi:10.1111/j.1551-2916.2005.00417.x.
- ↑ Barsoum, M.W. (2000). "The Mn+1AXn Phases: a New Class of Solids; Thermodynamically Stable Nanolaminates" (PDF). Prog. Solid State Chem. 28: 201–281.
- ↑ Barsoum, M.W. (2006) "Physical Properties of the MAX Phases" in Encyclopedia of Materials Science and Technology, K. H. J. Buschow (eds.). Elsevier, Amsterdam.
- ↑ Basu, Bikramjit; Kantesh Balani (2011). Advanced Structural Ceramics. Wiley. ISBN 0470497114.
- ↑ Music, D.; Schneider, J.M. (2007). "The Correlation between the Electronic Structure and Elastic Properties of Nanolaminates". JOM. 59: 60. doi:10.1007/s11837-007-0091-7.
- ↑ Emmerlich, J.; Music, D.; Braun, M.; Fayek, P.; Munnik, F.; Schneider, J.M. (2009). "A proposal for an unusually stiff and moderately ductile hard coating material: Mo2BC". Journal of Physics D: Applied Physics. 42: 185406. doi:10.1088/0022-3727/42/18/185406.
- ↑ Takahashi, T.; Music, D.; Schneider, J.M. (2012). "Influence of magnetic ordering on the elastic properties of PdFe3N". Journal of Vacuum Science and Technology A. 30: 030602.
- ↑ Magnuson, M. (2006). "Electronic structure and chemical bonding in Ti2AlC investigated by soft x-ray emission spectroscopy". Phys. Rev. B. 74 (19): 195108. arXiv:1111.2910
 . doi:10.1103/PhysRevB.74.195108.
. doi:10.1103/PhysRevB.74.195108. - ↑ Tallman, Darin J. (2013). "A Critical Review of the Oxidation of Ti2AlC, Ti3AlC2 and Cr2AlC in Air". Materials Research Letters. 1: 115. doi:10.1080/21663831.2013.806364.
- ↑ Magnuson, M. (2012). "The electronic-structure origin of the anisotropic thermopower of nanolaminated Ti3SiC2 determined by polarized x-ray spectroscopy and Seebeck measurements". Phys. Rev. B. 85 (19): 195134. arXiv:1205.4993
 . doi:10.1103/PhysRevB.85.195134.
. doi:10.1103/PhysRevB.85.195134. - ↑ Gilbert, C.J. (2000). "Fatigue-crack Growth and Fracture Properties of Coarse and Finegrained Ti3SiC2" (PDF). Scripta Materialia. 238 (2): 761–767.
- ↑ Guitton, A.; Joulain, A.; Thilly, L. & Tromas, C. (2014). "Evidence of dislocation cross-slip in MAX phase deformed at high temperature". Sci. Rep. 4: 6358. doi:10.1038/srep06358. PMC 4163670
 . PMID 25220949.
. PMID 25220949. - ↑ Barsoum, M.W. & El-Raghy, T. (1999). "Room Temperature Ductile Carbides". Metallurgical and Materials Trans. 30A (2): 363–369. Bibcode:1999MMTA...30..363B. doi:10.1007/s11661-999-0325-0.
- ↑ Barsoum, M.W., Farber, L., El-Raghy, T., & Levin, I. (1999). "Dislocations, Kink Bands and Room Temperature Plasticity of Ti3SiC2". Met. Mater. Trans. 30A (7): 1727–1738. doi:10.1007/s11661-999-0172-z.
- ↑ Guitton, A.; Joulain, A.; Thilly, L. & Tromas, C. (2012). "Dislocation analysis of Ti2AlN deformed at room temperature under confining pressure". Philosophical Magazine. 92 (36): 4536–4546. doi:10.1080/14786435.2012.715250.
- ↑ Guitton, A.; Van Petegem, S.; Tromas, C.; Joulain, A.; Van Swygenhoven, H. & Thilly, L. (2014). "Effect of microstructure anisotropy on the deformation of MAX polycrystals studied by in-situ compression combined with neutron diffraction". Applied Physics Letters. 104 (24): 241910. doi:10.1063/1.4884601.
- ↑ Farle, A (2016). "Demonstrating the self-healing behaviour of some selected ceramics under combustion chamber conditions". Smart Materials and Structures. 25: 1. doi:10.1088/0964-1726/25/8/084019.
- ↑ Tallman, Darin J. (2013). "A Critical Review of the Oxidation of Ti2AlC, Ti3AlC2 and Cr2AlC in Air". Materials Research Letters. 1: 115. doi:10.1080/21663831.2013.806364.
- ↑ Hoffman, Elizabeth (2012). "MAX phase carbides and nitrides: Properties for future nuclear power plant in-core applications and neutron transmutation analysis". Nuclear Engineering and Design. 244: 17. doi:10.1016/j.nucengdes.2011.12.009.
- ↑ Hoffman, Elizabeth (2008). "Micro and mesoporosity of carbon derived from ternary and binary metal carbides". Microporous and Mesoporous Materials. 112 (1-3): 526–532. doi:10.1016/j.micromeso.2007.10.033.
- ↑ Naguib, Michael (2011). "Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2". Advanced Materials. 23 (37): 4248–53. doi:10.1002/adma.201102306. PMID 21861270.