Membrane nanotube
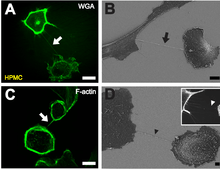
BDepiction of a NT (black arrow) between two cells with scanning electron microscopy one hour after cell plating. Scale bar: 10 µm.
C F-actin staining by fluorescently labeled phalloidin showing actin being present in NTs between individual HPMCs (white arrow). Scale bar: 20 µm.
D Scanning electron microscope picture of a substrate-associated filopodia-like extension as potential NT precursor (black arrowhead). The insert shows a fluorescence microscopic image of substrate associated filopodia-like protrusions approaching a neighboring cell (white arrowhead). Scale bar: 2 µm.
The term Membrane nanotubes, membrane nanotubules or cytoneme has been applied to protrusions that extend from the plasma membrane that enable different animal cells to touch over long distances, sometimes over 100 μm between T cells.[1][2]
Two types of structures have been called nanotubes. The first type are less than 0.7 micrometres in diameter, contain actin and carry portions of plasma membrane between cells in both directions. The second type are larger (>0.7 μm), contain both actin and microtubules and can carry components of the cytoplasm between cells, such as vesicles and organelles.[3]
These structures may be involved in cell-to-cell communication,[4] transfer of nucleic acids between cells in a tissue,[5] and the spread of pathogens or toxins such as HIV[1] and prions.[6] Membrane nanotubes were first described in a 1999 Cell article examining the development of Drosophila melanogaster wing imaginal discs.[7] More recently, a Science article published in 2004 described structures that connected various types of immune cell together, as well as connections between cells in tissue culture.[8][9]
Some dendritic cells (DC) and THP-1 monocytes have been shown to connect via Tunneling Nanotubules (TNT) and display evidence of calcium flux when exposed to bacterial or mechanical stimuli. TNT mediated signaling has shown to produce spreading in target cells, similar to the lamellipodia produced when DC cells are exposed to bacterial products. The TNT demonstrated in this study propagated at initial speed of 35 micrometers/second and have shown to connect THP-1 monocytes with nanotubules up to 100 micrometers long.[10]
Structures, called plasmodesmata, have been identified that do function as channels that interconnect plant cells[11] and stromules interconnect plastids.[12]
Vesicular transport in membrane nanotubes has been modeled utilizing a continuum approach.[13]
A variety of synthetic nanotubes, based on stacking of cyclic peptides and other cyclic molecules have been investigated.[14]
References
- 1 2 Sowinski S, Jolly C, Berninghausen O, et al. (February 2008). "Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission". Nat. Cell Biol. 10 (2): 211–9. doi:10.1038/ncb1682. PMID 18193035.
- ↑ Davis DM, Sowinski S (June 2008). "Membrane nanotubes: dynamic long-distance connections between animal cells". Nat. Rev. Mol. Cell Biol. 9 (6): 431–6. doi:10.1038/nrm2399. PMID 18431401.
- ↑ Onfelt B, Nedvetzki S, Benninger RK, et al. (December 2006). "Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria". J. Immunol. 177 (12): 8476–83. doi:10.4049/jimmunol.177.12.8476. PMID 17142745.
- ↑ Onfelt B, Davis DM (November 2004). "Can membrane nanotubes facilitate communication between immune cells?". Biochem. Soc. Trans. 32 (Pt 5): 676–8. doi:10.1042/BST0320676. PMID 15493985.
- ↑ Belting M, Wittrup A (December 2008). "Nanotubes, exosomes, and nucleic acid–binding peptides provide novel mechanisms of intercellular communication in eukaryotic cells: implications in health and disease". J. Cell Biol. 183 (7): 1187–91. doi:10.1083/jcb.200810038. PMC 2606965
 . PMID 19103810.
. PMID 19103810. - ↑ Gousset K, Schiff E, Langevin C, et al. (February 2009). "Prions hijack tunnelling nanotubes for intercellular spread". Nat. Cell Biol. 11 (3): 328–36. doi:10.1038/ncb1841. PMID 19198598.
- ↑ Ramírez-Weber FA, Kornberg TB (May 1999). "Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs". Cell. 97 (5): 599–607. doi:10.1016/S0092-8674(00)80771-0. PMID 10367889.
- ↑ Onfelt B, Nedvetzki S, Yanagi K, Davis DM (1 August 2004). "Cutting edge: Membrane nanotubes connect immune cells". J. Immunol. 173 (3): 1511–3. doi:10.4049/jimmunol.173.3.1511. PMID 15265877.
- ↑ Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH (February 2004). "Nanotubular highways for intercellular organelle transport". Science. 303 (5660): 1007–10. Bibcode:2004Sci...303.1007R. doi:10.1126/science.1093133. PMID 14963329.
- ↑ Simon C. Watkins, and Russell D. Salter (2005) "Functional Connectivity between Immune Cells Mediated by Tunneling Nanotubules" Immunity 23(3): 309-318. DOI: http://dx.doi.org/10.1016/j.immuni.2005.08.009
- ↑ Gallagher KL, Benfey PN (January 2005). "Not just another hole in the wall: understanding intercellular protein trafficking". Genes Dev. 19 (2): 189–95. doi:10.1101/gad.1271005. PMID 15655108.
- ↑ Köhler RH, Cao J, Zipfel WR, Webb WW, Hanson MR (1997). "Exchange of protein molecules through connections between higher plant plastids". Science. 276: 1039–1042. doi:10.1126/science.276.5321.2039. PMID 9197266.
- ↑ Kuznetsov, A.V. (2011). "Modeling bidirectional transport of quantum dot nanoparticles in membrane nanotubes". Mathematical Biosciences. 232 (2): 101–109. doi:10.1016/j.mbs.2011.04.008.
- ↑ Rodríguez-Vázquez, Nuria; Fuertes, Alberto; Amorín, Manuel; Granja, Juan R. (2016). "Chapter 14. Bioinspired Artificial Sodium and Potassium Ion Channels". In Astrid, Sigel; Helmut, Sigel; Roland K.O., Sigel. The Alkali Metal Ions: Their Role in Life. Metal Ions in Life Sciences. 16. Springer. pp. 485–556. doi:10.1007/978-4-319-21756-7_14.
External links
- Gurke S, Barroso JF, Gerdes HH (May 2008). "The art of cellular communication: tunneling nanotubes bridge the divide". Histochem. Cell Biol. 129 (5): 539–50. doi:10.1007/s00418-008-0412-0. PMC 2323029
 . PMID 18386044.
. PMID 18386044. - Hans-Hermann Gerdes Research Group - The laboratory that first observed membrane nanotubes
- <Please add first missing authors to populate metadata.>. "Tunnelling nanotubes: Life's secret network". New Scientist November 2008.