Fluorocarbon
Fluorocarbons, sometimes referred to as perfluorocarbons or PFCs, are, strictly speaking, organofluorine compounds with the formula CxFy, i.e. they contain only carbon and fluorine,[1] though the terminology is not strictly followed.[2] Compounds with the prefix perfluoro- are hydrocarbons, including those with heteroatoms, wherein all C-H bonds have been replaced by C-F bonds.[3] Fluorocarbons and their derivatives are useful fluoropolymers, refrigerants, solvents, and anesthetics.
Perfluoroalkanes
Chemical properties
Perfluoroalkanes are very stable because of the strength of the carbon–fluorine bond, one of the strongest in organic chemistry.[4] Its strength is a result of the electronegativity of fluorine imparting partial ionic character through partial charges on the carbon and fluorine atoms, which shorten and strengthen the bond through favorable covalent interactions. Additionally, multiple carbon–fluorine bonds increase the strength and stability of other nearby carbon–fluorine bonds on the same geminal carbon, as the carbon has a higher positive partial charge.[2] Furthermore, multiple carbon–fluorine bonds also strengthen the "skeletal" carbon–carbon bonds from the inductive effect.[2] Therefore, saturated fluorocarbons are more chemically and thermally stable than their corresponding hydrocarbon counterparts, and indeed any other organic compound. They are susceptible to attack by very strong reductants, e.g., Birch reduction and very specialized organometallic complexes.[5]
Fluorocarbons are colorless and have high density, up to over twice that of water. They are not miscible with most organic solvents (e.g., ethanol, acetone, ethyl acetate, and chloroform), but are miscible with some hydrocarbons (e.g., hexane in some cases). They have very low solubility in water, and water has a very low solubility in them (on the order of 10 ppm). They have low refractive indices.
The partial charges in the polarized carbon–fluorine bond |
As the high electronegativity of fluorine reduces the polarizability of the atom,[2] fluorocarbons are only weakly susceptible to the fleeting dipoles that form the basis of the London dispersion force. As a result, fluorocarbons have low intermolecular attractive forces and are lipophobic in addition to being hydrophobic and non-polar. Reflecting the weak intermolecular forces these compounds exhibit low viscosities when compared to liquids of similar boiling points, low surface tension and low heats of vaporization. The low attractive forces in fluorocarbon liquids make them compressible (low bulk modulus) and able to dissolve gas relatively well. Smaller fluorocarbons are extremely volatile.[2] There are five perfluoroalkane gases; tetrafluoromethane (bp −128 °C), hexafluoroethane (bp −78.2 °C), octafluoropropane (bp −36.5 °C), perfluoro-n-butane (bp −2.2 °C) and perfluoro-iso-butane (bp −1 °C). Nearly all other fluoroalkanes are liquids; the most notable exception is perfluorocyclohexane, which sublimes at 51 °C.[6] Fluorocarbons also have low surface energies and high dielectric strengths.[2]
- Perfluoroalkanes
-
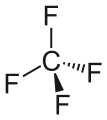
Carbon tetrafluoride, the simplest perfluoroalkane
-
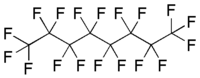
Perfluorooctane, a linear perfluoroalkane
-
.svg.png)
Perfluoro-2-methylpentane, a branched perfluoroalkane
-

Perfluoro-1,3-dimethylcyclohexane, a cyclic perfluoroalkane
-
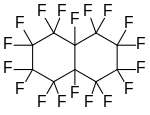
Perfluorodecalin, a polycyclic perfluoroalkane
Flammability
In the 1960s there was a lot of interest in fluorocarbons as anesthetics. The research did not come to anything, but a lot of effort was expended on the vital issue of flammability, and showed that the tested fluorocarbons were not flammable in air in any proportion, though most are in neat oxygen and neat nitrous oxide (gases of importance in anesthesiology).[7][8]
| Compound | Test conditions | Result |
|---|---|---|
| Hexafluoroethane | Lower flammability limit in oxygen | None |
| Perfluoropentane | Flash point in air | None |
| Flash point in oxygen | -6 °C | |
| Flash point nitrous oxide | -32 °C | |
| Perfluoromethylcyclohexane | Lower flammability limit in air | None |
| Lower flammability limit in oxygen | 8.3% | |
| Lower flammability limit in oxygen (50 °C) | 7.4% | |
| Lower flammability limit in nitrous oxide | 7.7% | |
| Perfluoro-1,3-dimethylcyclohexane | Lower flammability limit in oxygen (50 °C) | 5.2% |
| Perfluoromethyldecalin | Spontaneous ignition test in oxygen at 127 bar |
No ignition at 500 °C |
| Spontaneous ignition in adiabatic shock wave in oxygen, 0.98 to 186 bar |
No ignition | |
| Spontaneous ignition in adiabatic shock wave in oxygen, 0.98 to 196 bar |
Ignition |
Fluorocarbons have been considered as fire extinguishants to replace CFCs.[9] This extinguishing effect has been attributed to their high heat capacity, which takes heat away from the fire. It has been suggested that an atmosphere containing a significant percentage of perfluorocarbons on a space station or similar would prevent fires altogether.[10] [11] When combustion does occur, toxic fumes result, including carbonyl fluoride, carbon monoxide, and hydrogen fluoride.
Gas dissolving properties
Perfluorocarbons dissolve relatively high volumes of gases. The high solubility of gases is attributed to the weak intermolecular interactions in these fluorocarbon fluids.[12]
This table shows values for the mole fraction, x1, of nitrogen dissolved, calculated from the Ostwald coefficient, at 298.15 K (25 °C), 0.101325 M Pa.[13]
| Liquid | 104 x1 |
|---|---|
| Water | 0.118 |
| Ethanol | 3.57 |
| Acetone | 5.42 |
| Tetrahydrofuran | 5.21 |
| Cyclohexane | 7.73 |
| Perfluoromethylcyclohexane | 33.1 |
| Perfluoro-1,3-dimethylcyclohexane | 31.9 |
Manufacture
The development of the fluorocarbon industry coincided with World War II.[14] Prior to that, fluorocarbons were prepared by reaction of fluorine with the hydrocarbon, i.e., direct fluorination. Because C-C bonds are readily cleaved by fluorine, direct fluorination mainly affords smaller perfluorocarbons, such as tetrafluoromethane, hexafluoroethane, and octafluoropropane.[15]
Fowler process
A major breakthrough that allowed the large scale manufacture of fluorocarbons was the Fowler process. In this process, cobalt trifluoride is used as the source of fluorine. Illustrative is the synthesis of perfluorohexane:
- C6H14 + 28 CoF3 → C6F14 + 14 HF + 28 CoF2
The resulting cobalt difluoride is then regenerated, sometimes in a separate reactor:
- 2 CoF2 + F2 → 2 CoF3
Industrially, both steps are combined, for example in the manufacture of the Flutec range of fluorocarbons by F2 chemicals Ltd, using a vertical stirred bed reactor, with hydrocarbon introduced at the bottom, and fluorine introduced halfway up the reactor. The fluorocarbon vapor is recovered from the top.
Electrochemical fluorination
Electrochemical fluorination (ECF) (also known as the Simons' process) involves electrolysis of a substrate dissolved in hydrogen fluoride. As fluorine is itself manufactured by the electrolysis of hydrogen fluoride, ECF is a rather more direct route to fluorocarbons. The process proceeds at low voltage (5 - 6 V) so that free fluorine is not liberated. The choice of substrate is restricted as ideally it should be soluble in hydrogen fluoride. Ethers and tertiary amines are typically employed. To make perfluorohexane, trihexylamine is used, for example:
- 2 N(C6H13)3 + 90 HF → 6 C6F14 + 2 NF3 + 84 H2
The perfluorinated amine will also be produced:
- N(C6H13)3 + 39 HF → N(C6F13)3 + 39H2
Environmental and health concerns
Fluoroalkanes are generally very inert and non-toxic.[16][17][18]
They are not ozone depleting as they contain no chlorine or bromine atoms, and indeed they are sometimes used as replacements for ozone-depleting chemicals.[19] Unfortunately, the term fluorocarbon is used rather loosely to include any chemical containing fluorine and carbon, including chlorofluorocarbons, which are ozone depleting (for example here).
They are sometimes confused with fluorosurfactants, which have a significant bioaccumulation problem. However perfluoroalkanes do not bioaccumulate, and those used in medical procedures are rapidly excluded from the body, primarily via expiration. The rate of excretion appears to be a function of the vapour pressure; the half-life for octafluoropropane is less than 2 minutes,[20] compared to about a week for perfluorodecalin.[21]
Low-boiling perfluoroalkanes are potent greenhouse gases, in part due to their very long atmospheric lifetime. Their use is covered by the Kyoto Protocol.
A major source of atmospheric perfluorocarbons (tetrafluoromethane and hexafluoroethane especially) has been the aluminium smelting industry, where it is produced as a by-product of the electrolysis process.[22] However, the industry has been actively involved in reducing emissions in recent years.[23]
Applications
As they are inert, perfluoroalkanes have essentially no chemical uses, but their physical properties have led to their use in many diverse applications. These include:
- Perfluorocarbon tracer
- Liquid dielectric
- Chemical vapor deposition
- Organic Rankine cycle
- Fluorous biphasic catalysis [24]
- Cosmetics[25]
As well as several medical uses:
- Liquid breathing
- Blood substitute
- Contrast-enhanced ultrasound
- Eye surgery [26]
- Tattoo removal [27]
Fluoroalkenes and fluoroalkynes
Unsaturated fluorocarbons are far more reactive than fluoroalkanes. Although difluoroacetylene is unstable (as is typical for related alkynes, see dichloroacetylene),[2] hexafluoro-2-butyne and related fluorinated alkynes are well known.
- Unsaturated fluorocarbons
-
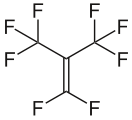
Perfluoroisobutene, a reactive and highly toxic fluoroalkene gas
-
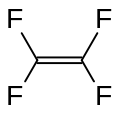
Tetrafluoroethylene, an important perfluorinated monomer.
-
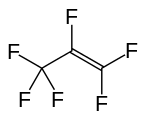
Hexafluoropropylene, another important perfluoroalkene.
-

Hexafluoro-2-butyne, a perfluoroalkyne.
Polymerization
Fluoroalkenes polymerize more exothermically than normal alkenes.[2] Unsaturated fluorocarbons have a driving force towards sp3 hybridization due to the electronegative fluorine atoms seeking a greater share of bonding electrons with reduced s character in orbitals.[2] The most famous member of this class is tetrafluoroethylene, which is used to manufacture polytetrafluoroethylene (PTFE), better known under the tradename Teflon.
Environmental and health concerns
Fluoroalkenes and fluorinated alkynes are reactive and many are toxic (perfluoroisobutene being a notable example). Fluoroalkenes are not ozone depleting, as they contain no chlorine or bromine atoms. They are too reactive to be greenhouse gases. Associated with the production of polytetrafluoroethylene is the use of various fluorinated surfactants, which have attracted concern because they bioaccumulate.
Perfluoroaromatic compounds
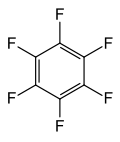
Perfluoroaromatic compounds contain only carbon and fluorine, like other fluorocarbons, but also contain an aromatic ring. The three most important examples are hexafluorobenzene, octafluorotoluene, and octafluoronaphthalene.
- Perfluoroaromatic compounds
Perfluoroaromatic compounds can be manufactured via the Fowler process, like fluoroalkanes, but the conditions must be adjusted to prevent full fluorination. They can also be made heating the corresponding perchloroaromatic compound with potassium fluorine at high temperature (typically 500 °C), during which the chlorine atoms are replaced by fluorine atoms. A third route is defluorination of the fluoroalkane; for example, octafluorotoluene can be made from perfluoromethylcyclohexane by heating to 500 °C with a nickel or iron catalyst.[28]
Perfluoroaromatic compounds are relatively volatile for their molecular weight, with melting and boiling points similar to the corresponding aromatic compound, as the table below shows. They have high density and are non-flammable. For the most part, they are clear, colorless liquids (octafluoronaphthalene, a yellow solid, is an exception). Unlike the perfluoralkanes, they tend to be miscible with common solvents.
| Compound | Melting point, °C | Boiling point, °C |
|---|---|---|
| Hexafluorobenzene | 5.3 | 80.5 |
| Benzene | 5.5 | 80.1 |
| Octafluorotoluene | <-70 | 102-103 |
| Toluene | -95 | 110.6 |
| Perfluoro(ethylbenzene) | - | 114-115 |
| Ethylbenzene | -93.9 | 136.2 |
| Octafluoronaphthalene | 86-87 | 209[29] |
| Naphthalene | 80.2 | 217.9 |
See also
- Category:Fluorocarbons
- Fluorochemical industry
- Fluorographene
References
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "fluorocarbons".
- 1 2 3 4 5 6 7 8 9 Lemal DM (January 2004). "Perspective on fluorocarbon chemistry". J. Org. Chem. 69 (1): 1–11. doi:10.1021/jo0302556. PMID 14703372.
- ↑ Murphy WJ (March 1947). "Fluorine Nomenclature... A statement by the Editors". Ind. Eng. Chem. 39 (3): 241–242. doi:10.1021/ie50447a004.
- ↑ O'Hagan D (February 2008). "Understanding organofluorine chemistry. An introduction to the C–F bond". Chem. Soc. Rev. 37 (2): 308–19. doi:10.1039/b711844a. PMID 18197347.
- ↑ Kiplinger JL, Richmond TG, Osterberg CE (1994). "Activation of Carbon-Fluorine Bonds by Metal Complexes". Chem. Rev. 94 (2): 373–431. doi:10.1021/cr00026a005.
- ↑ http://www.ornl.gov/~webworks/cpr/v823/rpt/108771.pdf
- ↑ Larsen ER (1969). "Fluorine Compounds in Anesthesiology: VI Flammability". Fluorine Chem. Rev. 3: 22–27.
- ↑ Flutec (Technical report). ISC Chemicals Limited. 1982.
- ↑ http://www.nist.gov/el/fire_research/upload/R9302960.pdf
- ↑ McHale ET (1974). "Life Support Without Combustion Hazards". Fire Technology. 10 (1): 15–24. doi:10.1007/bf02590509.
- ↑ Huggett C (1973). "Habitable Atmospheres Which Do Not Support Combustion". Combustion and Flame. 20: 140–142. doi:10.1016/s0010-2180(73)81268-4.
- ↑ http://www.f2chemicals.com/pdf/technical/Gas%20solubility.pdf
- ↑ Battino R, Rettich TR, Tominaga T (1984). "The solubility of nitrogen and air in liquids". J. Phys. Chem. Ref. Data. 13 (2): 308–19.
- ↑ McBee ET (March 1947). "Fluorine Chemistry". Ind. Eng. Chem. 39 (3): 236–237. doi:10.1021/ie50447a002.
- ↑ Siegemund G, Schwertfeger W, Feiring A, Smart B, Behr F, Vogel H, McKusick B "Fluorine Compounds, Organic" in "Ullmann's Encyclopedia of Industrial Chemistry" 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a11_349
- ↑ http://www.fluorocarbons.org/chemical-families/pfcs/pfc-toxicological-profile
- ↑ http://www.epa.gov/hpv/pubs/summaries/perfluro/c13244tp.pdf
- ↑ Yamanouchi K; Yokoyama K (1975). Proceedings of the Xth International Congress for Nutrition: Symposium on Perfluorochemical Artificial Blood, Kyoto: 91. Missing or empty
|title=(help) - ↑ http://www.expertglossary.com/climate-change/definition/perfluorocarbons-pfcs
- ↑ Platts DG; Fraser JF (2011). Critical Care and Resuscitation. 13 (1): 44–55. Missing or empty
|title=(help) - ↑ Geyer RP (1975). Proc. Xth Intern. Congress for Nutr.: Symp on Perfluorochemical Artif. Blood, Kyoto: 3–19. Missing or empty
|title=(help) - ↑ http://www.aluminum-production.com/anode_effect.html
- ↑ http://www.climatevision.gov/sectors/aluminum/pdfs/tmsfirst.pdf
- ↑ http://www.chemistry.illinois.edu/research/inorganic/seminar_abstracts/2002-2003/Flannigan.Abstract.LitSeminar.pdf
- ↑ http://www.beautymagonline.com/beauty-articles-4/1090-florinated-oils-2
- ↑ Imamura Y; Minami M; Ueki M; Satoh B; Ikeda T (2003). "Use of perfluorocarbon liquid during vitrectomy for severe proliferative diabetic retinopathy". Br J Ophthalmol. 87 (5): 563–566. doi:10.1136/bjo.87.5.563.
- ↑ http://www.aslms.org/professional/documents/EditorsChoiceLSMFeb45.2.pdf
- ↑ Banks, RE (1970). Fluorocarbons and their Derivatives, Second Edition. London: MacDonald & Co. (Publishers) Ltd. pp. 203–207. ISBN 0 356 02798 8.
- ↑ http://www.chemspider.com/Chemical-Structure.60886.html
External links
- Perfluorocycloalkene (PFCA)
- Fluorocarbons and Sulphur Hexafluoride, proposed by the European Fluorocarbons Technical Committee
- CFCs and Ozone Depletion Freeview video provided by the Vega Science Trust.
- Introduction to fluoropolymers
- Organofluorine chemistry by Graham Sandford