Protoplast

Protoplast, from ancient Greek πρωτόπλαστος (prōtóplastos, "first-formed"), in a religious context initially referred to the first human[1] or, more generally, to the first organized body of a species.
In biology, it was proposed by Hanstein (1880) to refer to the entire cell, excluding the cell wall,[2][3] but currently has several definitions:
- a plant, bacterial or fungal cell that had its cell wall completely or partially removed using either mechanical or enzymatic means. A further differentiation can be made for bacteria:
- protoplasts: have their cell wall entirely removed and are derived from gram + (gram-positive)
- spheroplasts: have their cell wall only partially removed and are gram - (gram-negative)
- more generally, a unit of biology which is composed of a cell's nucleus and the surrounding protoplasmic materials.
Enzymes for the preparation of protoplasts
Cell walls are made of a variety of polysaccharides. Protoplasts can be made by degrading cell walls with a mixture of the appropriate polysaccharide-degrading enzymes:
| Type of cell | Enzyme |
|---|---|
| Plant cells | Cellulase, pectinase, xylanase |
| Gram-positive bacteria | Lysozyme (+EDTA) |
| Fungal cells | Chitinase |
During and subsequent to digestion of the cell wall, the protoplast becomes very sensitive to osmotic stress. This means cell wall digestion and protoplast storage must be done in an isotonic solution to prevent rupture of the plasma membrane.
Uses for protoplasts
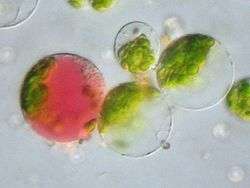
Protoplasts can be used to study membrane biology, including the uptake of macromolecules and viruses . These are also used in somaclonal variation.
Protoplasts are widely used for DNA transformation (for making genetically modified organisms), since the cell wall would otherwise block the passage of DNA into the cell.[4] In the case of plant cells, protoplasts may be regenerated into whole plants first by growing into a group of plant cells that develops into a callus and then by regeneration of shoots (caulogenesis) from the callus using plant tissue culture methods.[5] Growth of protoplasts into callus and regeneration of shoots requires the proper balance of plant growth regulators in the tissue culture medium that must be customized for each species of plant. Unlike protoplasts from vascular plants, protoplasts from mosses, such as Physcomitrella patens, do not need phytohormones for regeneration, nor do they form a callus during regeneration. Instead, they regenerate directly into the filamentous protonema, mimicking a germinating moss spore.[6]
Protoplasts may also be used for plant breeding, using a technique called protoplast fusion. Protoplasts from different species are induced to fuse by using an electric field or a solution of polyethylene glycol.[7] This technique may be used to generate somatic hybrids in tissue culture.
Additionally, protoplasts of plants expressing fluorescent proteins in certain cells may be used for Fluorescence Activated Cell Sorting (FACS), where only cells fluorescing a selected wavelength are retained. Among other things, this technique is used to isolate specific cell types (e.g., guard cells from leaves, pericycle cells from roots) for further investigations, such as transcriptomics.
See also
References
- ↑ The Apocalypse of Moses, http://www2.iath.virginia.edu/anderson/vita/english/vita.lat.html#per39
- ↑ Hanstein, J. 1880. Das Protoplasma. Heidelberg.
- ↑ Sharp, L. W. (1921). Introduction To Cytology. New York: McGraw Hill, p. 24.
- ↑ Davey MR, Anthony P, Power JB, Lowe KC (March 2005). "Plant protoplasts: status and biotechnological perspectives". Biotechnol. Adv. 23 (2): 131–71. doi:10.1016/j.biotechadv.2004.09.008. PMID 15694124.
- ↑ Thorpe TA (October 2007). "History of plant tissue culture". Mol. Biotechnol. 37 (2): 169–80. doi:10.1007/s12033-007-0031-3. PMID 17914178.
- ↑ S.C. Bhatla, Justine Kiessling, Ralf Reski (2002): of polarity induction by cytochemical localization of phenylalkylamine-binding receptors in regenerating protoplasts of the moss Physcomitrella patens. Protoplasma 219, 99–105.
- ↑ R. Hain and A.P. Czernilofsky et al. (1985). "Uptake, integration, expression and genetic transmission of a selectable chimaeric gene by plant protoplasts". Mol Gen Genet 199:161–168.