Sodium bismuthate
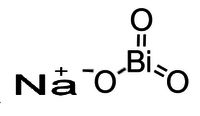 Idealized chemical formula of sodium bismuthate | |
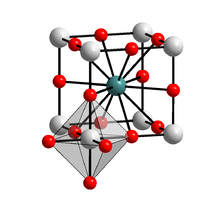 Solid state structure | |
| Names | |
|---|---|
| Other names
Sodium bismuth oxide | |
| Identifiers | |
| 12232-99-4 | |
| 3D model (Jmol) | Interactive image |
| ECHA InfoCard | 100.032.220 |
| EC Number | 235-455-6 |
| |
| |
| Properties | |
| NaBiO3 | |
| Molar mass | 279.97 g/mol |
| Appearance | Light brown powder |
| Density | 6.50 g/cm3 |
| insoluble in cold, decomposes in hot water | |
| Hazards | |
| EU classification (DSD) |
Harmful (Xn) |
| R-phrases | R22, R36/37/38 |
| S-phrases | S26, S36 |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
420 mg/kg (rat) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Sodium bismuthate is the inorganic compound with the formula NaBiO3. It is a yellowish solid that is a strong oxidiser.[1] It is not soluble in cold water, which is sometimes convenient since the reagent can be easily removed after the reaction. It is one of the few sodium salts that is insoluble in water. It is commercially available however commercial samples may be a mixture of bismuth(V) oxide, sodium carbonate and sodium peroxide.[2]
Structure
Sodium bismuthate adopts an ilmenite structure, consisting of octahedral Bi5+ centers and Na+ centers. The average Bi-O distance is 2.116 Å. The ilmenite structure is related to the corundum structure (Al2O3) with a layer structure formed by close packed oxygen atoms with the two different cations, Na+ and Bi5+ alternating in octahedral sites.[3]
Synthesis and reactions
Bismuth oxidizes to Bi(V) only with difficulty in the absence of alkali. For example, the simple oxide Bi2O5 remains poorly characterized. The preparation of this salt involves oxidizing a mixture of Bi2O3 and Na2O with air (source of O2):[4]
- Na2O + O2 + Bi2O3 → 2 NaBiO3
The procedure is analogous to the preparation oxidation of manganese dioxide in alkali to give sodium manganate.
It oxidizes water, decomposing into bismuth(III) oxide and sodium hydroxide:
- 2 NaBiO3 + H2O → 2 NaOH + Bi2O3 + O2
It is decomposed more rapidly by acids.
As a strong oxidizer, sodium bismuthate converts virtually any manganese compound to permanganate, which is easily assayed spectrophotometrically.[4] It is also used for lab-scale separation of plutonium.
 Vial of NaBiO3.
Vial of NaBiO3.
References
- ↑ "Sodium bismuthate". Mallinckrodt Baker. 06/19/07. Check date values in:
|date=(help) - ↑ Suzuki, Hitomi (2001). Organobismuth Chemistry. Elsevier. pp. 1–20. ISBN 978-0-444-20528-5.
- ↑ Kumada, N.; Kinomura, N.; Sleight, A.W. (November 2000). "Neutron powder diffraction refinement of ilmenite-type bismuth oxides: ABiO3 (A = Na, Ag)". Materials Research Bulletin. 35 (14-15): 2397–2402. doi:10.1016/S0025-5408(00)00453-0. ISSN 0025-5408. – via ScienceDirect (Subscription may be required or content may be available in libraries.)
- 1 2 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0-08-037941-9.