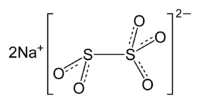
Sodium metabisulfite
 | |
 | |
| Names | |
|---|---|
| Other names
Sodium pyrosulfite Sodium disulfite | |
| Identifiers | |
| 7681-57-4 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:114786 |
| ChEMBL | ChEMBL2016976 |
| ECHA InfoCard | 100.028.794 |
| EC Number | 231-673-0 |
| E number | E223 (preservatives) |
| PubChem | 656671 |
| RTECS number | UX8225000 |
| |
| |
| Properties | |
| Na2S2O5, Na-O-(S=O)-O-(S=O)-O-Na | |
| Molar mass | 190.107 g/mol |
| Appearance | white to yellow powder |
| Odor | faint SO2 |
| Density | 1.48 g/cm3 |
| Melting point | 170 °C (338 °F; 443 K) decomposition begins at 150 °C |
| 45.1 g/100 mL (0 °C) 65.3 g/100 mL (20 °C) 81.7 g/100 mL (100 °C) | |
| Solubility | very soluble in glycerol soluble in ethanol |
| Hazards | |
| Safety data sheet | Mallinckrodt MSDS |
| EU classification (DSD) |
Harmful (Xn) Irritant (Xi) |
| R-phrases | R22 R31 R41 |
| S-phrases | (S2) S26 S39 S46 |
| NFPA 704 | |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
none[1] |
| REL (Recommended) |
TWA 5 mg/m3[1] |
| IDLH (Immediate danger) |
N.D.[1] |
| Related compounds | |
| Other anions |
Sodium sulfite Sodium bisulfite |
| Other cations |
Potassium metabisulfite |
| Related compounds |
Sodium dithionite Sodium thiosulfate Sodium sulfate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Sodium metabisulfite or sodium pyrosulfite (IUPAC spelling; Br. E. sodium metabisulphite or sodium pyrosulphite) is an inorganic compound of chemical formula Na2S2O5. The substance is sometimes referred to as disodium (metabisulfite). It is used as a disinfectant, antioxidant and preservative agent.
Preparation
Sodium metabisulfite can be prepared by evaporating a solution of sodium bisulfite saturated with sulfur dioxide:
- 2 HSO3− ⇌ H2O + S2O52−
which leaves solid Na2S2O5 behind.[2]
Chemical structure
The anion metabisulfite is a hybrid of dithionite (S2O42−) and dithionate (S2O62−). The anion consists of an SO2 group linked to an SO3 group, with the negative charge more localized on the SO3 end. The S–S bond length is 2.22 Å, and the "thionate" and "thionite" S–O distances are 1.46 and 1.50 Å respectively.[3]
Uses
Food additive
It is used as a preservative and antioxidant in food and is also known as E223.[4]
It may cause allergic reactions in those who are sensitive to sulfites, including respiratory reactions in asthmatics, anaphylaxis and other allergic reactions in sensitive individuals.[5][6]
Sodium metabisulfite and potassium metabisulfite are the primary ingredients in Campden tablets, used for wine and beer making.[7]
The acceptable daily intake is up to 0.7 milligrams per kilogram of body weight.[8] Sodium metabisulfite oxidizes in the liver to sulfate which is excreted in the urine.[8]
Sanitization and cleaning agent
It is commonly used in homebrewing and winemaking to sanitize equipment. It is used as a cleaning agent for potable water reverse osmosis membranes in desalination systems. It is also used to remove chloramine from drinking water after treatment.
Other uses
- Added to local anaesthetic (lidocaine etc.) solutions to prevent oxidation of vasoconstrictor adrenaline and thus improve the shelf life of the solution
- It is used in photography.[9]
- Concentrated sodium metabisulfite can be used to remove tree stumps. Some brands contain 98% sodium metabisulfite, and cause degradation of lignin in the stumps, facilitating removal.[10]
- It is also used as an excipient in some tablets, such as paracetamol. Approximately 0.5 mg is used in epinephrine autoinjectors such as the EpiPen.
- A very important health related aspect of this substance is that it can be added to a blood smear in a test for sickle cell anaemia (and other similar forms of haemoglobin mutation). The substances causes defunct cells to sickle (through a complex polymerisation) hence confirming disease.
- It is used as the source of SO2 in wine. An important anti-oxidant and bactericide
- It is also used to precipitate gold from auric acid (gold dissolved in aqua regia).
- It is used in waste treatment to chemically reduce hexavalent chromium to trivalent chromium which can then be precipitated and removed from an aqueous waste stream.
- It is used as a bleaching agent in the production of Coconut cream
- It is used as a reducing agent to break sulfide bonds in shrunken items of clothing made of natural fibers, thus allowing the garment to go back to its original shape after washing
- It is used as an SO2 source (mixed with air or oxygen) for the destruction of cyanide in commercial gold cyanidation processes.
- It is used in the oil and gas industry as a corrosion inhibitor/oxygen scavenger.
- It is used in the water treatment industry to quench chlorine residual
Chemical properties
When mixed with water, sodium metabisulfite releases sulfur dioxide (SO2), a pungent, unpleasant smelling gas that can also cause breathing difficulties in some people. For this reason, sodium metabisulfite has fallen from common use in recent times, with agents such as hydrogen peroxide becoming more popular for effective and odorless sterilization of equipment. Released sulfur dioxide however makes the water a strong reducing agent.
Sodium metabisulfite releases sulfur dioxide in contact with strong acids:
- Na2S2O5 + 2 HCl → 2 NaCl + H2O + 2 SO2
On heating to high temperature, it releases sulfur dioxide, leaving sodium sulfite behind:[11]
- Na2S2O5 → Na2SO3 + SO2
See also
References
- 1 2 3 "NIOSH Pocket Guide to Chemical Hazards #0566". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Catherine E. Housecroft; Alan G. Sharpe (2008). "Chapter 16: The group 16 elements". Inorganic Chemistry, 3rd Edition. Pearson. p. 520. ISBN 978-0-13-175553-6.
- ↑ K. L. Carter, T. A. Siddiquee, K. L. Murphy, D. W. Bennett "The surprisingly elusive crystal structure of sodium metabisulfite" Acta Crystallogr. (2004). B60, 155–162. doi:10.1107/S0108768104003325
- ↑ "Food Additives approved by the EU" (PDF). Retrieved 12 March 2015.
- ↑ Dean D. Metcalfe, Ronald A. Simon, Food allergy: adverse reactions to food and food additives, Wiley-Blackwell 2003, p. 324–339
- ↑ http://www.ific.org/publications/brochures/asthmabroch.cfm
- ↑ Milne, George W. A. (2005). Gardner's commercially important chemicals: synonyms, trade names, and properties. New York: Wiley-Interscience. p. 568. ISBN 0-471-73518-3.
- 1 2 "Food-Info.net : E-numbers : E223: Sodium disulphite". food-info.net.
- ↑ Anchell, Steve (2008). The darkroom cookbook (3rd ed.). Amsterdam: Focal Press. p. 193. ISBN 0240810554.
- ↑ http://www.bonideproducts.com/lbonide/msds/msds271.pdf
- ↑ Sodium sulfite. http://www.chemicalbook.com/ChemicalProductProperty_EN_cb4111698.htm
External links
- International Chemical Safety Card 1461
- The surprisingly elusive crystal structure of sodium metabisulfite
- CDC - NIOSH Pocket Guide to Chemical Hazards
