Sodium iodate
 | |
| Names | |
|---|---|
| Other names
Iodic acid, sodium salt | |
| Identifiers | |
| 7681-55-2 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 22760 |
| ECHA InfoCard | 100.028.793 |
| EC Number | 231-672-5 |
| PubChem | 23675764 |
| RTECS number | NN1400000 |
| |
| |
| Properties | |
| INaO3 | |
| Molar mass | 197.89 g·mol−1 |
| Appearance | White orthorhombic crystals |
| Odor | Odorless |
| Density | 4.28 g/cm3 |
| Melting point | 425 °C (797 °F; 698 K) (anhydrous) decomposes[1] 19.85 °C (67.73 °F; 293.00 K) (pentahydrate) |
| 2.5 g/100 mL (0 °C) 8.98 g/100 mL (20 °C) 9.47 g/100 mL (25 °C)[2] 32.59 g/100 mL (100 °C)[3] | |
| Solubility | Soluble in acetic acid Insoluble in alcohol |
| Solubility in dimethylformamide | 0.5 g/kg[2] |
| Structure | |
| Orthorhombic | |
| Thermochemistry | |
| 125.5 J/mol·K[2] | |
| Std molar entropy (S |
135 J/mol·K[2] |
| Std enthalpy of formation (ΔfH |
−490.4 kJ/mol[2] |
| Gibbs free energy (ΔfG˚) |
35.1 kJ/mol[2] |
| Hazards | |
| GHS pictograms | 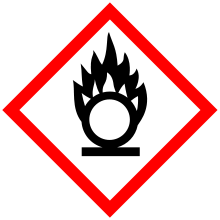  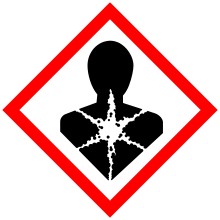 [4] [4] |
| GHS signal word | Danger |
| H272, H302, H317, H334[4] | |
| P220, P261, P280, P342+311[4] | |
| EU classification (DSD) |
|
| R-phrases | R8, R22, R42/43 |
| S-phrases | S17, S22, S36/37, S45 |
| NFPA 704 | |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
108 mg/kg (mice, intravenous)[2] |
| Related compounds | |
| Other anions |
Sodium iodide Sodium periodate Sodium bromate Sodium chlorate |
| Other cations |
Potassium iodate Silver iodate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Sodium iodate (NaIO3) is the sodium salt of iodic acid. Sodium iodate is an oxidizing agent, and as such it can cause fires upon contact with combustible materials or reducing agents.
Preparation
It can be prepared by reacting a sodium-containing base such as sodium hydroxide with iodic acid, for example:
It can also be prepared by adding iodine to a hot, concentrated solution of sodium hydroxide or its carbonate:
Reactions
Sodium iodate can be oxidized to sodium periodate in water solutions by hypochlorites or other strong oxidizing agents:
Safety
Conditions/substances to avoid are: heat, shock, friction, combustible materials, reducing materials, aluminium, organic compounds, carbon, hydrogen peroxide, sulfides.
References
- ↑ Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, FL: CRC Press. pp. 4–85. ISBN 0-8493-0594-2.
- 1 2 3 4 5 6 7 http://chemister.ru/Database/properties-en.php?dbid=1&id=759
- ↑ Seidell, Atherton; Linke, William F. (1919). Solubilities of Inorganic and Organic Compounds (2nd ed.). D. Van Nostrand Company.
Results here are multiplied by water's density at temperature of solution for unit conversion. - 1 2 3 Sigma-Aldrich Co., Sodium iodate. Retrieved on 2014-05-25.
This article is issued from Wikipedia - version of the 6/9/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.
