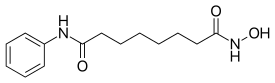
Vorinostat
 | |
| Clinical data | |
|---|---|
| Pronunciation | vor-IN-oh-stat |
| Trade names | Zolinza |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607050 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Oral (capsules) |
| ATC code | L01XX38 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 1.8–11%[1] |
| Protein binding | ~71% |
| Metabolism |
Hepatic glucuronidation and β-oxidation CYP system not involved |
| Metabolites | vorinostat O-glucuronide, 4-anilino-4-oxobutanoic acid (both inactive)[2] |
| Biological half-life | ~2 hours (vorinostat and O-glucuronide), 11 hours (4-anilino-4-oxobutanoic acid) |
| Excretion | Renal (negligible) |
| Identifiers | |
| |
| CAS Number |
149647-78-9 |
| PubChem (CID) | 5311 |
| IUPHAR/BPS | 6852 |
| DrugBank |
DB02546 |
| ChemSpider |
5120 |
| UNII |
58IFB293JI |
| KEGG |
D06320 |
| ChEBI |
CHEBI:45716 |
| ChEMBL |
CHEMBL98 |
| ECHA InfoCard | 100.207.822 |
| Chemical and physical data | |
| Formula | C14H20N2O3 |
| Molar mass | 264.32 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Vorinostat (rINN)[3] also known as suberanilohydroxamic acid (suberoyl+anilide+hydroxamic acid abbreviated as SAHA) is a member of a larger class of compounds that inhibit histone deacetylases (HDAC). Histone deacetylase inhibitors (HDI) have a broad spectrum of epigenetic activities.
Vorinostat is marketed under the name Zolinza (zo-LINZ-ah) by Merck for the treatment of cutaneous manifestations in patients with cutaneous T cell lymphoma (CTCL) when the disease persists, gets worse, or comes back during or after two systemic therapies.[2][4] The compound was developed by Columbia University chemist Ronald Breslow and Memorial Sloan-Kettering researcher Paul Marks.[5][6]
Medical uses
Vorinostat was the first histone deacetylase inhibitor[7] approved by the U.S. Food and Drug Administration (FDA) for the treatment of CTCL on October 6, 2006.[8] It also failed to demonstrate efficacy in treating acute myeloid leukemia in a phase II study.[9]
Mechanism of action
Vorinostat has been shown to bind to the active site of histone deacetylases and act as a chelator for zinc ions also found in the active site of histone deacetylases.[10] Vorinostat's inhibition of histone deacetylases results in the accumulation of acetylated histones and acetylated proteins, including transcription factors crucial for the expression of genes needed to induce cell differentiation.[10]
Clinical trials
Vorinostat has also been used to treat Sézary syndrome, another type of lymphoma closely related to CTCL.[11]
A recent study suggested that vorinostat also possesses some activity against recurrent glioblastoma multiforme, resulting in a median overall survival of 5.7 months (compared to 4–4.4 months in earlier studies).[12] Further brain tumor trials are planned in which vorinostat will be combined with other drugs.
Including vorinostat in treatment of advanced non-small-cell lung carcinoma (NSCLC) showed improved response rates and increased median progression free survival and overall survival.[13]
It has given encouraging results in a phase II trial for myelodysplastic syndromes in combination with idarubicin and cytarabine.[14]
Preclinical investigations
Vorinostat is an interesting target for scientists interested in eradicating HIV from infected persons.[15] Vorinostat was recently shown to have both in vitro and in vivo effects against latently HIV infected T cells.[16][17]
Vorinostat also has shown some activity against the pathophysiological changes in α1-antitrypsin deficiency[18] and cystic fibrosis.[19] Recent evidences also indicate Vorinostat as a therapeutic tool for Niemann-Pick type C1 (NPC1), a rare lysosomal lipid storage disease.[20]
See also
References
- ↑ "Withdrawal Assessment Report for Vorinostat MSD 100 mg Hard Capsules (vorinostat)" (PDF). European Medicines Agency. 23 October 2008. p. 9. Retrieved 1 September 2016.
- 1 2 "Zolinza (vorinostat) Capsules. Full Prescribing Information" (PDF). Merck & Co., Inc., Whitehouse Station, NJ 08889, USA. Retrieved 1 September 2016.
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List 56" (PDF). WHO Drug Information. 20 (3): 232. 2006. Retrieved 1 September 2016.
- ↑ "ZOLINZA, Merck's Investigational Medicine for Advanced Cutaneous T-Cell Lymphoma (CTCL), To Receive Priority Review from U.S. Food and Drug Administration" (Press release). Merck & Co. June 7, 2006. Retrieved 2006-10-06.
- ↑ Lee, J.-H.; Mahendran, A.; Yao, Y.; Ngo, L.; Venta-Perez, G.; Choy, M. L.; Kim, N.; Ham, W.-S.; Breslow, R.; Marks, P. A. (2013). "Development of a histone deacetylase 6 inhibitor and its biological effects". Proceedings of the National Academy of Sciences. 110 (39): 15704–9. Bibcode:2013PNAS..11015704L. doi:10.1073/pnas.1313893110. PMC 3785767
 . PMID 24023063.
. PMID 24023063. - ↑ Marks, Paul A; Breslow, Ronald (2007). "Dimethyl sulfoxide to vorinostat: development of this histone deacetylase inhibitor as an anticancer drug". Nature Biotechnology. 25 (1): 84–90. doi:10.1038/nbt1272. PMID 17211407.
- ↑ HDAC Inhibitors Base (vorinostat)
- ↑ "Zolinza (vorinostat) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 16 February 2014.
- ↑ Schaefer, E. W.; Loaiza-Bonilla, A.; Juckett, M.; DiPersio, J. F.; Roy, V.; Slack, J.; Wu, W.; Laumann, K.; Espinoza-Delgado, I.; Gore, S. D. (2009). "A phase 2 study of vorinostat in acute myeloid leukemia". Haematologica. 94 (10): 1375–82. doi:10.3324/haematol.2009.009217. PMC 2754953
 . PMID 19794082.
. PMID 19794082. - 1 2 Richon, V M (2006). "Cancer biology: mechanism of antitumour action of vorinostat (suberoylanilide hydroxamic acid), a novel histone deacetylase inhibitor". British Journal of Cancer. 95 (Suppl 1): S2–S6. doi:10.1038/sj.bjc.6603463. PMC 2360770
 .
. - ↑ Cuneo A, Castoldi. "Mycosis fungoides/Sezary's syndrome". Retrieved 2008-02-15.
- ↑ "Vorinostat shows anti-cancer activity in recurrent gliomas" (Press release). Mayo Clinic. June 3, 2007. Retrieved 2007-06-03.
- ↑ Ramalingam, S. S.; Maitland, M. L.; Frankel, P.; Argiris, A. E.; Koczywas, M.; Gitlitz, B.; Thomas, S.; Espinoza-Delgado, I.; Vokes, E. E.; Gandara, D. R.; Belani, C. P. (2010). "Carboplatin and Paclitaxel in Combination With Either Vorinostat or Placebo for First-Line Therapy of Advanced Non-Small-Cell Lung Cancer". Journal of Clinical Oncology. 28 (1): 56–62. doi:10.1200/JCO.2009.24.9094. PMC 2799233
 . PMID 19933908.
. PMID 19933908. - ↑ "Zolinza, Idarubicin, Cytarabine Combination Yields High Response Rates In MDS Patients (ASH 2011)".
- ↑ Clinical trial number NCT01319383 for "The Effect of Vorinostat on HIV RNA Expression in the Resting CD4+ T Cells of HIV+ Pts on Stable ART" at ClinicalTrials.gov
- ↑ Archin, Nancie M.; Espeseth, Amy; Parker, Daniel; Cheema, Manzoor; Hazuda, Daria; Margolis, David M. (2009). "Expression of Latent HIV Induced by the Potent HDAC Inhibitor Suberoylanilide Hydroxamic Acid". AIDS Research and Human Retroviruses. 25 (2): 207–12. doi:10.1089/aid.2008.0191. PMC 2853863
 . PMID 19239360.
. PMID 19239360. - ↑ Contreras, X.; Schweneker, M.; Chen, C.-S.; McCune, J. M.; Deeks, S. G.; Martin, J.; Peterlin, B. M. (2009). "Suberoylanilide Hydroxamic Acid Reactivates HIV from Latently Infected Cells". Journal of Biological Chemistry. 284 (11): 6782–9. doi:10.1074/jbc.M807898200. PMC 2652322
 . PMID 19136668.
. PMID 19136668. - ↑ Bouchecareilh, M.; Hutt, D. M.; Szajner, P.; Flotte, T. R.; Balch, W. E. (2012). "Histone Deacetylase Inhibitor (HDACi) Suberoylanilide Hydroxamic Acid (SAHA)-mediated Correction of 1-Antitrypsin Deficiency". Journal of Biological Chemistry. 287 (45): 38265–78. doi:10.1074/jbc.M112.404707. PMC 3488095
 . PMID 22995909.
. PMID 22995909. - ↑ Hutt, Darren M; Herman, David; Rodrigues, Ana P C; Noel, Sabrina; Pilewski, Joseph M; Matteson, Jeanne; Hoch, Ben; Kellner, Wendy; Kelly, Jeffery W; Schmidt, Andre; Thomas, Philip J; Matsumura, Yoshihiro; Skach, William R; Gentzsch, Martina; Riordan, John R; Sorscher, Eric J; Okiyoneda, Tsukasa; Yates, John R; Lukacs, Gergely L; Frizzell, Raymond A; Manning, Gerard; Gottesfeld, Joel M; Balch, William E (2010). "Reduced histone deacetylase 7 activity restores function to misfolded CFTR in cystic fibrosis". Nature Chemical Biology. 6 (1): 25–33. doi:10.1038/nchembio.275. PMC 2901172
 . PMID 19966789.
. PMID 19966789. - ↑ Alam, M. S.; Getz, M.; Haldar, K. (2016). "Chronic administration of an HDAC inhibitor treats both neurological and systemic Niemann-Pick type C disease in a mouse model". Science Translational Medicine. 8 (326): 326ra23. doi:10.1126/scitranslmed.aad9407. PMID 26888431.
External links
- Vorinostat bound to proteins in the PDB