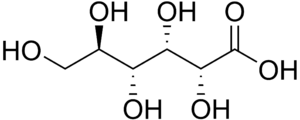
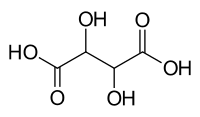
Sugar acid
Sugar acids are monosaccharides with a carboxyl group.[1]
Main classes of sugar acids include:
- Aldonic acids, in which the aldehyde functional group of an aldose is oxidized
- Ulosonic acids, in which the first hydroxyl group of a 2-ketose is oxidised creating an α-ketoacid.
- Uronic acids, in which the terminal hydroxyl group of an aldose or ketose is oxidized
- Aldaric acids, in which both ends of an aldose are oxidized
Examples
Examples of sugar acids include:
- Aldonic acids
- Glyceric acid (3C)
- Xylonic acid (5C)
- Gluconic acid (6C)
- Ascorbic acid[2] (6C, unsaturated lactone)
- Ulosonic acids
- Neuraminic acid (5-amino-3,5-dideoxy-D-glycero-D-galacto-non-2-ulosonic acid)
- Ketodeoxyoctulosonic acid (KDO or 3-deoxy-D-manno-oct-2-ulosonic acid)
- Uronic acids
- Glucuronic acid (6C)
- Galacturonic acid (6C)
- Iduronic acid (6C)
- Aldaric acids
- Tartaric acid (4C)
- meso-Galactaric acid (Mucic acid) (6C)
- D-Glucaric acid (Saccharic acid) (6C)
 The β-D form of glucuronic acid |
References
- ↑ Robyt, J.F. (1998). Essentials of carbohydrate chemistry. New York: Springer. ISBN 0-387-94951-8.
- ↑ Davies Michael B.; Austin John; Partridge David A. (1991). Vitamin C: Its Chemistry and Biochemistry. The Royal Society of Chemistry. p. 48. ISBN 0-85186-333-7.
External links
- Sugar Acids at the US National Library of Medicine Medical Subject Headings (MeSH)
This article is issued from Wikipedia - version of the 11/7/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.