Thorselliaceae
| Thorselliaceae | |
|---|---|
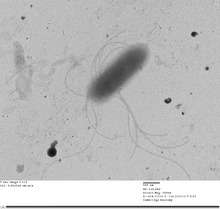 | |
| Thorsellia anophelis | |
| Scientific classification | |
| Kingdom: | Bacteria |
| Phylum: | Proteobacteria |
| Class: | Gammaproteobacteria |
| Order: | ? |
| Family: | Thorselliaceae Kämpfer et al., 2015 |
| Genus: | Thorsellia |
| Species | |
|
T. anophelis | |
Thorselliaceae is a family of bacteria belonging to the class Gammaproteobacteria and it was first described in February 2015.[1] The family consists of three described species. The bacteria are Gram-negative and rod shaped, approximately 1 μm wide and 2 μm long. They are facultative anaerobes and motile. Thorselliaceae bacteria have been found around the world associated with vector mosquitoes, mainly with vectors of malaria.
The first described species was Thorsellia anophelis.[2] It was isolated from the midgut of the malaria mosquito Anopheles arabiensis from Kenya.[3] This new bacterium was given its name after the Swedish researcher Walborg Thorsell who worked with mosquitoes and insecticides against mosquitoes for many years. Thorsellia bacteria have now been found in mosquito species that are the major vectors of malaria in Africa, Asia and South America. The bacteria have also been found in the waters where the malaria mosquitoes breed. Some properties of Thorsellia suggest that they are adapted to the mosquito guts, they can accept an alkaline pH, which is found in mosquito larvae and they grow faster in blood culture.[4] However, T. anophelis has also been found in the reproductive tissues of male and female Anopheles gambiae and An. coluzzii mosquitoes.[5]
Currently, little is known about Thorselliaceae in nature apart from that it has been shown in several studies that Thorsellia anophelis dominate the malaria mosquito gut flora and their breeding waters.[4][6][7]
Thorsellia has besides in Anopheles also been found to dominate the gut flora in the mosquito species Culex tarsalis,[8][9] which is a vector of among other things, West Nile virus and encephalitis.
Application
A possible application of Thorselliaceae is in paratransgenesis to prevent the transmission of malaria. This would involve genetically transforming the bacteria with genes that produce effector molecules against malaria parasites inside the malaria mosquito gut.[10] Thorselliaceae can be grown in the laboratory under normal conditions and are related to the bacterium Escherichia coli which suggests that the molecular techniques needed could be similar to those available for E. coli.
References
- ↑ Kämpfer, P; Glaeser, SP; Nilsson, LKJ; Eberhard, T; Håkansson, S; Guy, L; Roos, S; Busse, HJ; Terenius, O (2015). "Proposal of Thorsellia kenyensis sp. nov. and Thorsellia kandunguensis sp. nov., isolated from the larvae of Anopheles arabiensis as members of the family Thorselliaceae fam. nov". Int J Syst Evol Microbiol. 65 (Pt 2): 444–451. doi:10.1099/ijs.0.070292-0. PMID 25385997.
- ↑ Kämpfer, P; Lindh, JM; Terenius, O; Haghdoost, S; Falsen, E; Busse, HJ; Faye, I (2006). "Thorsellia anophelis gen. nov., sp. nov., a new member of the Gammaproteobacteria". Int J Syst Evol Microbiol. 56 (Pt 2): 335–338. doi:10.1099/ijs.0.63999-0.
- ↑ Lindh, JM; Terenius, O; Faye, I (2005). "16S rRNA gene-based identification of midgut bacteria from field-caught Anopheles gambiae sensu lato and A. funestus mosquitoes reveals new species related to known insect symbionts". Appl Environ Microbiol. 71 (11): 7217–7223. doi:10.1128/aem.71.11.7217-7223.2005.
- 1 2 Briones, AM; Shililu, J; Githure, J; Novak, R; Raskin, L (2008). "Thorsellia anophelis is the dominant bacterium in a Kenyan population of adult Anopheles gambiae mosquitoes". The ISME Journal. 2 (1): 74–82. doi:10.1038/ismej.2007.95.
- ↑ Segata, Nicola; Baldini, Francesco; Pompon, Julien; Garrett, Wendy S.; Truong, Duy Tin; Dabiré, Roch K.; Diabaté, Abdoulaye; Levashina, Elena A.; Catteruccia, Flaminia (2016-04-18). "The reproductive tracts of two malaria vectors are populated by a core microbiome and by gender- and swarm-enriched microbial biomarkers". Scientific Reports. 6. doi:10.1038/srep24207. ISSN 2045-2322.
- ↑ Wang, Y; Gilbreath, TM III; Kukutla, P; Yan, G; Xu, J (2011). "Dynamic gut microbiome across life history of the malaria mosquito Anopheles gambiae in Kenya". PLOS ONE. 6 (9): e24767. doi:10.1371/journal.pone.0024767. PMC 3177825
 . PMID 21957459.
. PMID 21957459. - ↑ Buck, M; Nilsson, LKJ; Brunius, C; Dabiré, RK; Hopkins, R; Terenius, O (2016-03-10). "Bacterial associations reveal spatial population dynamics in Anopheles gambiae mosquitoes". Scientific Reports. 6. doi:10.1038/srep22806. PMC 4785398
 . PMID 26960555.
. PMID 26960555. - ↑ Duguma, D; Rugman-Jones, P; Kaufman, MG; Hall, MW; Neufeld, JD; Stouthamer, R; Walton, WE (2013). "Bacterial communities associated with culex mosquito larvae and two emergent aquatic plants of bioremediation importance". PLOS ONE. 15 (8(8)): e72522. doi:10.1371/journal.pone.0072522.
- ↑ Duguma, Dagne; Hall, Michael W.; Rugman-Jones, Paul; Stouthamer, Richard; Terenius, Olle; Neufeld, Josh D.; Walton, William E. (2015). "Developmental succession of the microbiome of Culex mosquitoes". BMC Microbiology. 15 (1): 140. doi:10.1186/s12866-015-0475-8. ISSN 1471-2180. PMC 4513620
 . PMID 26205080.
. PMID 26205080. - ↑ Wang, S; Jacobs-Lorena, M (2013). "Genetic approaches to interfere with malaria transmission by vector mosquitoes". Trends Biotechnol. 31 (3): 185–193. doi:10.1016/j.tibtech.2013.01.001. PMID 23395485.