Hyperandrogenism
| Hyperandrogenism | |
|---|---|
|
| |
| Testosterone is a type of androgen that is important in the development of hyperandrogenism since high levels of it can cause this condition. | |
| Pronunciation | 'hīpər'andrəjənizəm |
| Classification and external resources | |
| Specialty | Endocrinology |
| ICD-10 | E25, E28.1 |
| ICD-9-CM | 255.2 |
| MedlinePlus | 001165 |
| eMedicine | article/273153 |
Hyperandrogenism, also known as androgen excess, is a medical condition characterized by excessive levels of androgens (male sex hormones such as testosterone) in the female body and the associated effects of the elevated androgen levels. It is an endocrinological disorder similar to hyperestrogenism. The most common conditions associated with hyperandrogenism are polycystic ovary syndrome or PCOS, a set of symptoms caused by androgen excess in females, and various cancers that can cause androgen excess. In females, the condition usually present are some combination of acne, seborrhea (inflamed skin), hair loss on the scalp, increased body and/or facial hair (hirsutism), and an elevated sex drive or libido. The symptoms of hyperandrogenism are usually most effectively treated with antiandrogens. There is some controversy over whether hyperandrogenism provides an unfair advantage in athletics.
Signs and symptoms
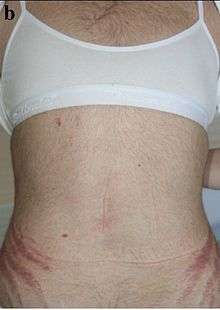
Hyperandrogenism affects 5-10% of females of reproductive age.[1] Hyperandrogenism can affect both males and females, but is more noticeable in females due to the fact that elevated levels of androgens in females often facilitates virilization. Due to the fact that hyperandrogenism is characterized by the elevation of male sex hormone levels, symptoms of hyperandrogenism in men are often negligible. Hyperandrogenism in females is typically diagnosed in late adolescence with a medical evaluation. The medical evaluation tends to consist of a pelvic exam, observation of external symptoms, and a blood test measuring androgen levels.[2]
- Hirsutism - male-pattern hair growth
- Alopecia - balding
- Masculine appearance
- Hidradenitis suppurativa
- Polycystic ovarian syndrome
- Oligomenorrhea - menstrual irregularities
- Acne
- Obesity
- Infertility
- Deepening of voice
- Oily skin
- Seborrhea - skin inflammation
- Libido - increased sex drive
- Type 2 diabetes
Effects of hyperandrogenism
In women
Hyperandrogenism, especially high levels of testosterone, can cause serious adverse effects on women’s bodies if left untreated. High testosterone levels have been seen to be associated with obesity, hypertension, amenorrhea(stop of menstrual cycles), and ovulatory dysfunction, which can lead to infertility. The more prominent signs of hyperandrogenism are hirsutism(unwanted growth of hair especially in the abdominal region and places on the back), acne after adolescents, deepening of voice, and alopecia(balding).[3] Hyperandrogenism has also been seen to cause individuals to have a high tolerance to insulin, which can lead to type two diabetes, and dyslipidemia, such as high cholesterol. These effects have also been seen to have a large psychological impact on the individual, sometimes often leading to societal anxiety and depression, especially in adolescent girls and young women. Paired with obesity and hirsutism, it can cause the individual to have low self-esteem, and a poor view of oneself.[2][4]
In men
Even though hyperandrogenism is not common in men, there has been studies done to look at the effects of high levels of testosterone in male bodies. A study have shown that even though many of the male participates did not have a behavior changes due to the increased levels of testosterone, there were cases where the participants had instances of uncharacteristic aggression. High levels of testosterone in male has not been seen to have a direct impact on their personality, but within those studies, there have been cases of sudden aggression within the male participants.[5]
Causes
While hyperandrogenism in women is caused by external factors, it can also appear from natural causes.
Non-tumoral
Polycystic ovary syndrome
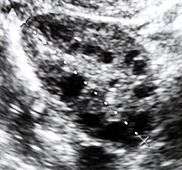
Polycystic ovary syndrome (PCOS) is an endocrine disorder characterized by an excess of androgens produced by the ovaries. It is estimated that approximately 90 percent of women with PCOS demonstrate hypersecretion of these hormones.[6] A concrete cause for this condition is currently unknown. Speculations include genetic predisposition, although the gene or genes in particular have yet to be identified.[7] Evidence suggests that the condition may have a hereditary basis. Other possible causes include the effects from an increase in insulin production. Insulin itself has been observed capable of inducing excess testosterone levels in the ovaries.[8] Elevated insulin concentration in the body leads to lower production of sex hormone binding globulin (SHBG), a regulatory glycoprotein that suppresses the function of androgens.[9] High blood levels of insulin also work in conjunction with ovarian sensitivity to insulin to cause hyperandrogenemia, the primary symptom of PCOS. Obese individuals may be more biologically inclined to display PCOS due to markedly higher amounts of insulin in their bodies. This hormonal imbalance can lead to chronic anovulation, in which the ovaries experiences difficulty releasing mature eggs. These cases of ovulatory dysfunction are linked to infertility and menstrual disturbances.[6][10]
Hyperthecosis and hyperinsulinemia
Hyperthecosis occurs when the cells of the ovarian stroma transition from interstitial cells, which are cells located in between other cells, into luteinized theca cells. Theca cells are located in the ovarian follicles and become luteinized when the ovarian follicle breaks and a new corpus luteum is formed. The dispersal of luteinized theca cells throughout the ovarian stroma, in contrast to PCOS where the luteinized theca cells are only around cystic follicles, causes women with hyperthecosis to have higher testosterone levels and male-attributed characteristics (virilization) than women with PCOS. Excess levels of insulin in the blood, known as hyperinsulinemia, is also a characteristic of hyperthecosis.[11] Hyperthecosis is mostly seen in postmenopausal women and is linked to acne, hirsutism, growth of the clitoris, baldness, and voice deepening.[12]
Low levels of insulin can also lead to hyperandrogenism. When the body’s insulin levels drop too low, it can force itself to produce too much in an effort to make up for the loss. The result of such an overproduction is a disorder called hyperinsulinemia. An effect of hyperinsulinemia is the body’s increased production of androgens in the ovaries.[13] This is all part of HAIR-AN syndrome, a multisystem disorder that involves increased insulin levels that prompt increased androgen levels.[14][15]
Cushing’s syndrome
Cushing syndrome develops due to long-term exposure to the hormone cortisol. Cushing’s syndrome can either be exogenous or endogenous, depending on whether it is caused by an external or internal source, respectively. The intake of glucocorticoids, which are a type of steroid hormone, is a common cause for the development of exogenous Cushing’s syndrome. Endogenous Cushing’s syndrome can occur when the body produces excessive amounts of cortisol. This occurs when the hypothalamus of the brain transmits corticotropin-releasing hormone (CRH) to the pituitary gland, which in turn secretes adrenocorticotropin hormone (ACTH). ACTH causes the adrenal glands to then release cortisol into the blood. Signs of Cushing’s syndrome include muscle weakness, easy bruising, weight gain, male-pattern hair growth (hirsutism), colored stretch marks, and excess of reddish complexion in the face.[16] Cushing’s syndrome has been shown to cause androgen excess, which directly links it to the signs and symptoms seen in hyperandrogenism.[12]
Congenital adrenal hyperplasia
Congenital Adrenal Hyperplasia consists of a group of autosomal recessive disorders that cause a lack of an enzyme needed for producing cortisol and/or aldosterone, both of which are steroid hormones. Most cases of CAH are due to 21-hydroxylase deficiencies, an enzyme used by the body to produce cortisol and aldosterone. In females, CAH causes uncertainty in the genitals at birth and later on in adolescence excessive pubic hair, enlargement of the clitoris, hirsutism, and rapid growth of the body. Symptoms in males include early showings of pubic hair, enlargement of the penis, and rapid body and skeletal growth.[17]
Tumors
Adrenal tumors
Adrenocortical carcinoma and tumors
A highly uncommon disease with incidence of 1–2 per million annually. This disease causes cancerous cells to form in the cortex of one or both of the adrenal glands. Adrenocortical tumors produce an additional number of hormones, often leading patients with steroid hormone-producing tumors to develop Cushing's syndrome, Conn syndrome and Hyperandrogenism.[18]
Adenoma of the adrenal gland
Adrenal Adenomas are benign tumors on the adrenal gland. In most cases the tumors display no symptoms and require no treatment. In rare cases, however, some Adrenal Adenomas may become activated, in that they begin to produce hormones in much larger quantities than what adrenal glands tend to produce leading to a number of health complications including Primary aldosteronism and Hyperandrogenism.[19]
Ovarian tumors
Arrhenoblastoma
An arrhenoblastoma is an uncommon tumor of the ovary. It is often composed of sterol cells, ludig cells or some combination of the two. The tumor can produce male or female hormones in the patient and may cause masculinization. In a prepubescent child, a tumor may cause precocious puberty. Malignant Arrhenoblastoma accounts for 30% of all cases of Arrhenoblastoma, the other 70% being largely benign and curable with surgery.[20]
Hilar cell tumor
An ovarian, Androgen producing tumor afflicting older women in most cases and often leading to the development of virilization. This tumor tends to occur around the region of the ovary where the blood vessels enter the organ otherwise known as the hilum. This type of tumor tends to be rather small in size and in most cases could be entirely removed and its symptoms reversed through surgery.[21]
Krukenberg tumor
A quickly developing malignant tumor that is normally found in one of or both ovaries. The tumor is caused by the transcoelomic spread. It primarily grows in the stomach and intestinal regions.[22]
Menopause
One such cause is the end of ovulation and the beginning of menopause. When the body transitions from ovulation to menopause, it stops releasing estrogen at faster rate than it stops releasing androgens. In some cases, estrogen levels can drop enough that there are substantially higher androgen levels leading to hyperandrogenism. A decrease in sex hormone levels while the free androgen index increases helps to aid this process, as well.[23]
Drug-induced hyperandrogenism
Symptoms generally considered hyperandrogenic can also manifest as results of consuming certain drugs. This can happen according to one of five major mechanisms, namely the direct introduction of androgens to the body, the binding of the drug to androgen receptors and subsequent participation in androgenic action (as is the case with anabolic-androgenic steroids), the reduction of sex hormone-binding globulin plasma concentration that leads to a resulting increase in free testosterone, the interference with and alteration of the hypothalamic–pituitary–ovarian (HPO) axis, or the increase in release of adrenal androgens.[24]
Heredity
Because hyperandrogenism can appear as a symptom of numerous different genetic and medical conditions, it is difficult to make a general statement on whether hyperandrogenic symptoms can be passed from parent to offspring. However, a collection of the conditions with hyperandrogenic symptoms, including polycystic ovary syndrome, have been observed as hereditary in certain cases. One potential cause of polycystic ovary syndrome is maternal hyperandrogenism, where the hormonal irregularities of the mother can affect the development of the child during gestation, resulting in the passing of polycystic ovary syndrome from mother to child.[25]
Diagnosis
Female patients may show symptoms of hyperandrogenism in their early life, but physicians become more concerned when the patient is in her late teens or older.[2]
Hyperandrogenism is most often diagnosed by checking for signs of hirsutism according to a standardized method that scores the range of excess hair growth.[1][2]
Checking medical history and a physical examination of symptoms are used for an initial diagnosis.[2] Patient history assessed includes age at thelarche, adrenarche, and menarche; patterns of menstruation; obesity; reproductive history; and the start and advancement of hyperandrogenism symptoms.[2] Patterns of menstruation are examined since irregular patterns may appear with hirsutism.[1] Family history is also assessed for occurrences of hyperandrogenism symptoms or obesity in other family members.[2]
A laboratory test can also be done on the patient to evaluate levels of FSH, LH, DHEAS, prolactin, 17OHP, and total and free testosterone in the patient's blood.[2] Abnormally high levels of any of these hormones help in diagnosing hyperandrogenism.[2]
Prevention
Since etiological factors are not known and vary among individuals with hyperandrogegism, there is no sure method to prevent this medical condition.[26] Therefore, more longterm studies are needed first to find a cause for the condition before being able to find a sufficient method of prevention.[26]
However, there are a few things that can help avoid long-term medical issues related to hyperandrogenism like PCOS. Getting checked by a medical professional for hyperandrogenism; especially if one has a family history of the condition, irregular periods, or diabetes; can be beneficial.[27] Watching your weight and diet is also important in decreasing your chances, especially in obese females, since continued exercise and maintaining a healthy diet leads to an improved menstrual cycle as well as to decreased insulin levels and androgen concentrations.[26]
Treatment and management
Treatment of hyperandrogenism varies with the underlying condition that causes it. As a hormonal symptom of polycystic ovary syndrome, menopause, and other endocrine disorders, it is primarily treated as a symptom of these disorders. Systemically, it is treated with antiandrogens such as cyproterone acetate, flutamide and spironolactone to control the androgen levels in the patient's body. For Hyperandrogenism caused by Late-Onset Congenital Adrenal Hyperplasia (CAH), treatment is primarily focused on providing the patient with Glucocorticoids to combat the low cortisol production and the corresponding increase in androgens caused by the swelling of the Adrenal Glands.[28][29] Oestrogen-based oral contraceptives are used to treat both CAH and PCOS caused hyperandrogenism. These hormonal treatments have been found to reduce the androgen excess and suppress adrenal androgen production and cause a significant decrease in hirstutism.[30][31]
Hyperandrogenism is often managed symptomatically. Hirsutism and acne both respond well to the hormonal treatments described above, with 60-100% reporting an improvement in Hirstutism.[30] Androgenic alopecia however, does not show a significant improvement with hormonal treatments and requires other treatments, such as hair transplantation.[32]
Culture and society
Because androgen excess is manifested in noticeable physical features (ex. hirsutism), a certain social stigma is associated with it. In the athletic world, multiple cases of female athletes being banned for their testosterone levels being too high have been recorded. Such social and cultural redefinitions of hyperandrogenism are important to consider outside of the clinical usage.
Sports
Following the case of South African athlete Caster Semenya, the International Association of Athletics Federations (IAAF) introduced a now suspended policy to exclude women athletes from competing as women if they have hyperandrogenism, on the ground that the condition could confer an unfair advantage.[33][34] The rules state that women may compete in the male category if their performance qualifies.[35] The IAAF states that testosterone is linked to lean body mass(LBM), so it influences athletes' strength, speed and power.[36]
The permissible testosterone limit was set at 10 nmol/L, based on a study of women competing in the World Championships in 2011 and 2013.[37] 99% of the female athletes at those competitions had testosterone levels below 3.08 nmol/L.[37] This upper limit of 10 nmol/L was more than 3 times higher than the testosterone levels of 99% of the elite female athletes in those competitions. However, a study of endocrine profiles in 693 elite athletes published in 2014 found that 13.7% of women athletes had high levels of testosterone, and 16.5% of men had low levels of testosterone levels. The authors noted that there is "complete overlap between the sexes", concluding, "The IOC definition of a woman as one who has a ‘normal’ testosterone level is untenable."[38][39]
The test has been controversial, with suggestions that it is discriminatory.[40] There is evidence that women from developing countries have been subjected to partial clitoridectomies and gonadectomies following test results revealing hyperandrogenism.[41] In September 2014, Dutee Chand, a sprinter from India who was barred by the IAAF from competing against other female runners, sought to appeal the ruling and asked for reinstatement.[42] In July 2015, the Court of Arbitration for Sport suspended the IAAF ban, thus reinstating Chand's right to compete. The IAAF was given two years in which to file scientific evidence justifying the ban. In the absence of evidence, the ban will be declared void.[43][44][45]

The suspension of the IAAF test for hyperandrogenism led to controversy in the Rio 2016 Olympic Games,[46] in particular related to the participation and performance of South African middle distance runner Caster Semenya. Competitors Lynsey Sharp and Joanna Jóźwik spoke out about their belief that Semenya has a competitive advantage, Jóźwik reportedly claimed that she was the "first European" and "second white" to finish the race.[47] Many bioethicists and gender equality advocates argue that preventing women with higher levels of testosterone from participating is a form of discrimination, penalizing the athlete for a natural trait of her body, much akin to the natural advantage possessed by taller basketball players or marathoners who train at higher altitudes.[46][48]
Social definition
Cultural variation can define hyperandrogenism socially—aside from clinical and chemical definitions—to make some hair growth unacceptable even if it is considered clinically normal based on metrics like the Ferriman-Gallwey score. For example, only pubic and axillary hair in North American women is tolerated, while other androgen-dependent hair such as growth on the upper lip, over the linea alba, over the thighs, and any periareolar hair is not.[49]
Organizations
Professional organizations like the Androgen Excess and PCOS Society exist to promote the research, treatment, diagnosis, and prevention of such disorders along with educating the public and scientific community about them.[50]
See also
- Hypoandrogenism
- Hypergonadism
- Hypogonadism
- Hyperestrogenism
- Hypoestrogenism
- Androgen-dependent condition
References
- 1 2 3 Yildiz, Bulent O. (June 2006). "Diagnosis of hyperandrogenism: clinical criteria". Best Practice & Research Clinical Endocrinology & Metabolism. 20 (2): 167–176. doi:10.1016/j.beem.2006.02.004. ISSN 1521-690X. Retrieved 13 November 2016.
- 1 2 3 4 5 6 7 8 9 Goodman, Neil (March 2001). American association of clinical endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of hyperandrogenic disorders. p. 121.
- ↑ Simon, James (2015-06-22). "Androgen". Health Women. National Women's Health Resource Center. Retrieved 2016-11-14.
- ↑ Brettenthaler, Nora; De Geyter, Christian; R. Huber, Peter; Keller, Ulrich (April 21, 2004). "Effect of the Insulin Sensitizer Pioglitazone on Insulin Resistance, Hyperandrogenism, and Ovulatory Dysfunction in Women with Polycystic Ovary Syndrome". Endocrine Society. Endocrine Society (published April 28, 2011). Retrieved 2016-11-14.
- ↑ G. Pope Jr., Harrison; M. Kouri, Elena; I.Hudson, James (March 5, 1999). "Effects of Supraphysiologic Doses of Testosterone on Mood and Aggression in Normal Men". The JAMA Network. American Medical Association. Retrieved 2016-11-14.
- 1 2 Franks, Stephen. "Polycystic Ovary Syndrome — NEJM." New England Journal of Medicine. N Engl J Med, 28 Sept. 1995. Web. 14 Nov. 2016.
- ↑ "Polycystic Ovary Syndrome (PCOS)." Causes. Mayo Clinic, n.d. Web. 09 Nov. 2016.
- ↑ "Defining PCOS." - The University of Chicago Medicine. The University of Chicago Medical Center, n.d. Web. 10 Nov. 2016.
- ↑ Hammond GL, Bocchinfuso WP (1996). "Sex hormone-binding globulin: gene organization and structure/function analyses". Hormone Research. 45 (3-5): 197–201
- ↑ Burd, Irina, David Zieve, and Isla Ogilvie. "Polycystic Ovary Syndrome: MedlinePlus Medical Encyclopedia." Polycystic Ovary Syndrome: MedlinePlus Medical Encyclopedia. A.D.A.M Inc., n.d. Web. 09 Nov. 2016.
- ↑ Pasquali, Renato (April 2011). "Research in Polycystic Ovary Syndrome Today and Tomorrow". Medscape. Blackwell Publishing. Retrieved 14 November 2016.
- 1 2 Atmaca, Murat (16 December 2014). "An Interesting Cause of Hyperandrogenemic Hirsutism". Case Reports in Endocrinology.
- ↑ Barbieri; Hornstein MD. ""Hyperinsulinemia and Ovarian Hyperandrogenism. Cause and Effect."". Endocrinology and Metabolism Clinics of North America. 17.4: 685–703.
- ↑ James, William; Berger, Timothy; Elston, Dirk (2005). Andrews' Diseases of the Skin: Clinical Dermatology. (10th ed.). Saunders.
- ↑ Somani N, Harrison S, Bergfeld WF (2008). "The clinical evaluation of hirsutism". Dermatol Ther. 21 (5): 376–91
- ↑ "Cushing's Syndrome". National Institute of Diabetes and Digestive and Kidney Diseases. April 2012. Retrieved 14 November 2016.
- ↑ Wilson, Thomas (23 June 2016). "Congenital Adrenal Hyperplasia". Medscape. Retrieved 14 November 2016.
- ↑ "Adrenocortical Carcinoma." National Cancer Institute. N.p., n.d. Web. 10 Nov. 2016.
- ↑ "Adenoma of the Adrenal Gland | Genetic and Rare Diseases Information Center(GARD) – an NCATS Program." U.S National Library of Medicine. U.S. National Library of Medicine, n.d. Web. 10 Nov. 2016.
- ↑ Martin, Elizabeth. Arrhenoblastoma N. Arrhenoblastoma. n.p.: Oxford University Press, 2015. Oxford Reference. Web. 10 Nov. 2016
- ↑ Martin, Elizabeth. "hilar cell tumour." Concise Medical Dictionary. : Oxford University Press, 2015. Oxford Reference. 2015. Date Accessed 10 Nov. 2016
- ↑ Uyeturk, Ummugul, Serife Hulya Arslan, Oznur Bal, Ulku Yalcintas Arslan, and Omur Berna Cakmak Oksuzoglu. "Isolated Ovarian Metastasis of Gastric Cancer: Krukenberg Tumor." Contemporary Oncology. Termedia Publishing House, 19 Dec. 2013. Web. 10 Nov. 2016.
- ↑ "R. H. Fogle, F. Z. Stanczyk, X. Zhang, and R. J. Paulson, "Ovarian androgen production in postmenopausal women"". Journal of Clinical Endocrinology and Metabolism. 92 (8, pp. 3040– 3043, 2007.).
- ↑ Neraud, Barbara; Dewailly, Didier. Contemporary Endocrinology: Androgen Excess Disorders in Women: Polycystic Ovary Syndrome and Other Disorders, Second Edition. Edited by: R. Azziz et al. © Humana Press Inc., Totowa, NJ.
- ↑ "Puttabyatappa Muraly, Cardoso Rodolfo C., Padmanabhan Vasantha. In Special issue: Impact of maternal metabolism on newborn health". Molecular and Cellular Endocrinology. 5 November 2016 435:29-39.
- 1 2 3 Apter, D. "How Possible Is The Prevention Of Polycystic Ovary Syndrome Development In Adolescent Patients With Early Onset Of Hyperandrogenism." Journal Of Endocrinological Investigation 21.9 (1998): 613-617. MEDLINE. Web. 15 Nov. 2016.
- ↑ Nader, Shahla. "Hyperandrogenism During Puberty In The Development Of Polycystic Ovary Syndrome." Fertility And Sterility 1 (2013): AGRIS. Web. 15 Nov. 2016.
- ↑ I. A. Hughes. "Management of Congenital Adrenal Hyperplasia". Archives of Disease in Childhood. 1988;63:1399-1404
- ↑ D. Merke, S. R. Bornstein. (2005). Congenital adrenal hyperplasia. In: Lancet. doi:10.1016/S0140-6736(05)66736-0
- 1 2 Burkman R.T. Jr. The Role of Oral Contraceptives in the Treatment of Hyperandrogenic Disorders. AM J Med. 1995 Jan 16;98(1A):130S-136S.
- ↑ Mastorakos George, Koliopoulos Carolina, Creatsas George. Androgen and lipid profiles in adolescents with polycystic ovary syndrome who were treated with two forms of combined oral contraceptives. Fertility and Sterility. May 2002; 77(5):919-927.
- ↑ Sinclair R, Wewerinke M, Jolley D.Treatment of female pattern hair loss with oral antiandrogens. Br J Dermatol. 2005 Mar;152(3):466-73
- ↑ "STANDARD OPERATIVE PROCEDURE to identify Circumstances (Female Hyperandrogenism) in Which A Particular Sports Person will not be eligible to participate in Competitions in the Female Category".
- ↑ http://pib.nic.in/archieve/others/2013/mar/d2013032001.pdf
- ↑ International Olympic Committee (November 2015), IOC Consensus Meeting on Sex Reassignment and Hyperandrogenism (PDF)
- ↑ "The biggest issue in women's sports is about to come to a head". Mother Jones. Retrieved 2016-11-12.
- 1 2 "Hyperandrogenism and women vs women vs men in sport: A Q&A with Joanna Harper".
- ↑ Healy, M. L.; Gibney, J.; Pentecost, C.; Wheeler, M. J.; Sonksen, P. H. (August 2014). "Endocrine profiles in 693 elite athletes in the postcompetition setting". Clinical Endocrinology. 81 (2): 294–305. doi:10.1111/cen.12445. ISSN 0300-0664. Retrieved 2016-08-17.
- ↑ Stewart, Erin (August 17, 2016). "Haters and hyperandrogenism: Caster Semenya's road to becoming an Olympic favourite". SBS. Retrieved 2016-08-17.
- ↑ Padawer, Ruth (2016-06-28). "The Humiliating Practice of Sex-Testing Female Athletes". The New York Times. ISSN 0362-4331. Archived from the original on 2016-06-28. Retrieved 2016-06-28.
- ↑ Jordan-Young, R. M.; Sonksen, P. H.; Karkazis, K. (April 2014). "Sex, health, and athletes". BMJ. 348 (apr28 9): –2926–g2926. doi:10.1136/bmj.g2926. ISSN 1756-1833. Retrieved 2016-05-21.
- ↑ "Fighting for the Body She Was Born With". The New York Times. 7 October 2014.
- ↑ "Dutee Chand, Female Sprinter With High Testosterone Level, Wins Right to Compete". The New York Times. 28 July 2015.
- ↑ http://www.tas-cas.org/fileadmin/user_upload/Media_Release_3759_FINAL.pdf
- ↑ "Dutee Chand cleared to race as IAAF suspends 'gender test' rules". BBC News Online. 27 July 2015. Retrieved 27 July 2015.
- 1 2 REID, SCOTT M. "Caster Semenya's Olympic dream a topic of controversy". The Orange County Register. Retrieved 2016-11-10.
- ↑ "Fifth-placed runner behind Semenya 'feels like silver medalist' and glad she was the 'second white'". The Independent. 2016-08-22. Retrieved 2016-11-10.
- ↑ Longman, Jeré (2016-08-18). "Understanding the Controversy Over Caster Semenya". The New York Times. ISSN 0362-4331. Retrieved 2016-11-10.
- ↑ Krishna; R, Usha (2000-01-01). Adolescent Gynecology (pb). Orient Blackswan. ISBN 9788125017936.
- ↑ wang, stephanie qian. "Androgen Excess and PCOS Society". www.ae-society.org. Retrieved 2016-11-10.