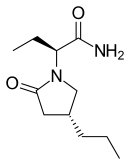
Brivaracetam
 | |
| Clinical data | |
|---|---|
| Pronunciation | BRIV-a-RA-se-tam |
| Trade names | Briviact |
| AHFS/Drugs.com | Briviact |
| Pregnancy category |
|
| Routes of administration | Oral (tablets, oral solution), IV |
| ATC code | N03AX23 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Nearly 100% |
| Protein binding | ≤20% |
| Metabolism | Hydrolysis by amidase, CYP2C19-mediated hydroxylation |
| Metabolites | 3 inactive metabolites |
| Biological half-life | ~9 hours |
| Excretion | Kidneys (>95%)[1] |
| Identifiers | |
| |
| CAS Number |
357336-20-0 |
| PubChem (CID) | 9837243 |
| ChemSpider |
8012964 |
| UNII |
U863JGG2IA |
| KEGG | D08879 |
| ChEBI |
CHEBI:133013 |
| ChEMBL |
CHEMBL607400 |
| ECHA InfoCard | 100.118.642 |
| Chemical and physical data | |
| Formula | C11H20N2O2 |
| Molar mass | 212.15 g/mol |
| 3D model (Jmol) | Interactive image |
| Specific rotation | [α]D −60° |
| Melting point | 72 to 77 °C (162 to 171 °F) |
| |
| |
Brivaracetam (trade name Briviact), a chemical analog of levetiracetam, is a racetam derivative with anticonvulsant (antiepileptic) properties.[2][3] It is marketed by the pharmaceutical company UCB.
Medical uses
Brivaracetam is used to treat partial-onset seizures with or without secondary generalisation, in combination with other antiepileptic drugs. No data are available for its effectiveness and safety in patients younger than 16 years.[4][5]
Adverse effects
The most common adverse effects include sleepiness, dizziness, nausea and vomiting. More rarely, coordination problems and changes in behaviour can occur.[4][5]
Interactions
Coadministration of brivaracetam with carbamazepine may increase exposure to carbamazepine-epoxide, the active metabolite of carbamazepine, and could theoretically lead to reduced tolerability. Coadministration of brivaracetam with phenytoin may increase phenytoin levels. Coadministration of other antiseizure drugs are unlikely to affect brivaracetam exposure. Brivaracetam provides no added therapeutic benefit when administered in conjunction with levetiracetam that acts on the same protein.[6]
Pharmacology
Mechanism of action
Brivaracetam is believed to act by binding to the ubiquitous synaptic vesicle glycoprotein 2A (SV2A), like levetiracetam. but with 20-fold greater affinity.[7][8] There is some evidence that racetams including levetiracetam and brivaracetam access the luminal side of recycling synaptic vesicles during vesicular endocytosis. They may reduce excitatory neurotransmitter release and enhance synaptic depression during trains of high-frequency activity, such as is believed to occur during epileptic activity. [9]
Pharmacokinetics
Brivaracetam exhibits linear pharmacokinetics over a wide dose range, is rapidly and completely absorbed after oral administration, has an elimination half-life of 7 to 8 hours, and has plasma protein binding of less than 20%. It is extensively metabolized (>90%), primarily via hydrolysis of the acetamide group, and secondarily through hydroxylation mediated by the liver enzyme CYP2C19. The three major metabolites (hydroxy, acid, and hydroxyacid) are pharmacologically inactive. Brivaracetam is eliminated as urinary metabolites, with over 95% of a radioactive test dose recovered in the urine within 72 hours, including only 8.6% as unchanged brivaracetam.[10]
Chemical and physical properties
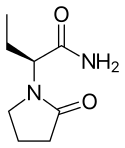
Brivaracetam is the 4R-propyl analogue of the anticonvulsant levetiracetam.
History
Positive preliminary results from stage III trials were recorded in 2008,[11] along with evidence that it is around 10 times more potent for the prevention of certain types of seizure in mouse models than its analogue levetiracetam.[12]
On 14 January 2016, the European Commission,[5] and on 18 February 2016, the Food and Drug Administration[13] approved brivaracetam under the trade name Briviact. The Drug Enforcement Administration (DEA) has issued an interim final rule placing brivaracetam into schedule V of the Controlled Substances Act (CSA) effective 12 May 2016.[14] As of May 2016, brivaracetam is not approved in other countries, including Australia, Canada and Switzerland.
References
- ↑ "Briviact (brivaracetam) Tablets, for Oral Use; Oral Solution; Injection, for Intravenous Use. CV. Full Prescribing Information" (PDF). UCB, Inc., Smyrna, GA 30080. Retrieved 27 August 2016.
- ↑ von Rosenstiel P (Jan 2007). "Brivaracetam (UCB 34714)". Neurotherapeutics. 4 (1): 84–7. doi:10.1016/j.nurt.2006.11.004. PMID 17199019.
- ↑ Malawska B, Kulig K (Jul 2005). "Brivaracetam UCB". Current Opinion in Investigational Drugs. 6 (7): 740–746. PMID 16044671.
- 1 2 Drugs.com: briviact for Briviact.
- 1 2 3 "Briviact". European Medicines Agency. Retrieved 30 May 2016.
- ↑ Rolan P, Sargentini-Maier ML, Pigeolet E, Stockis A (2008). "The pharmacokinetics, CNS pharmacodynamics and adverse event profile of brivaracetam after multiple increasing oral doses in healthy men". Br J Clin Pharmacol. 66 (1): 71–5. doi:10.1111/j.1365-2125.2008.03158.x. PMC 2485265
 . PMID 18341673.
. PMID 18341673. - ↑ Rogawski MA, Bazil CW (Jul 2008). "New molecular targets for antiepileptic drugs: alpha(2)delta, SV2A, and K(v)7/KCNQ/M potassium channels". Current Neurology and Neuroscience Reports. 8 (4): 345–352. doi:10.1007/s11910-008-0053-7. PMC 2587091
 . PMID 18590620.
. PMID 18590620. - ↑ Haberfeld, H, ed. (2015). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag.
- ↑ Rogawski MA (2016). "A new SV2A ligand for epilepsy". Cell. 167: 587. doi:10.1016/j.cell.2016.09.057.
- ↑ Sargentini-Maier ML, Espié P, Coquette A, Stockis A (2008). "Pharmacokinetics and metabolism of 14C-brivaracetam, a novel SV2A ligand, in healthy subjects". Drug Metab Dispos. 36 (1): 36–45. doi:10.1124/dmd.107.017129. PMID 17908923.
- ↑ Rogawski MA (Aug 2008). "Brivaracetam: a rational drug discovery success story". British Journal of Pharmacology. 154 (8): 1555–7. doi:10.1038/bjp.2008.221. PMC 2518467
 . PMID 18552880.
. PMID 18552880. - ↑ Matagne A, Margineanu DG, Kenda B, Michel P, Klitgaard H (Aug 2008). "Anti-convulsive and anti-epileptic properties of brivaracetam (ucb 34714), a high-affinity ligand for the synaptic vesicle protein, SV2A". British Journal of Pharmacology. 154 (8): 1662–71. doi:10.1038/bjp.2008.198. PMC 2518465
 . PMID 18500360.
. PMID 18500360. - ↑ "FDA approves Briviact to treat partial onset seizures". US FDA. 19 February 2016.
- ↑ Drug Enforcement Administration Department of Justice (May 12, 2016). "Schedules of Controlled Substances: Placement of Brivaracetam Into Schedule V. Interim final rule, with request for comments.". Fed Regist. 81 (92): 29487–92. PMID 27192732.