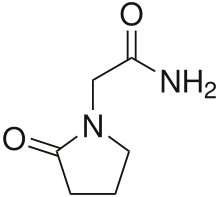
Piracetam
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Breinox, Dinagen, Lucetam, Nootropil, Nootropyl, Oikamid, Piracetam and many others |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral, parenteral, or vaporized |
| ATC code | N06BX03 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~100% |
| Biological half-life | 4–5 hr |
| Excretion | Urinary |
| Identifiers | |
| |
| CAS Number |
7491-74-9 |
| PubChem (CID) | 4843 |
| IUPHAR/BPS | 4288 |
| ChemSpider |
4677 |
| UNII |
ZH516LNZ10 |
| KEGG |
D01914 |
| ChEMBL |
CHEMBL36715 |
| ECHA InfoCard | 100.028.466 |
| Chemical and physical data | |
| Formula | C6H10N2O2 |
| Molar mass | 142.16 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Piracetam (sold under many brand names) is a nootropic drug in the racetams group, with chemical name 2-oxo-1-pyrrolidine acetamide. It shares the same 2-oxo-pyrrolidone base structure with pyroglutamic acid. Piracetam is a cyclic derivative of GABA. Presently piracetam is used in many European countries, Asia and South America. In the United States, it is not approved by the US Food and Drug Administration for any medical use and it is not permitted to be sold as a dietary supplement.[1][2] In the UK, piracetam is prescribed mainly for myoclonus,[3] but is used off-label for other conditions. Evidence to support its use for many conditions is unclear.
Medical uses
Dementia
A 2001 Cochrane review concluded that there was not enough evidence to support piracetam for dementia or cognitive problems.[4] A 2002 review and 2005 review concluded that piracetam had some positive effects in older patients with these problems.[5][6] In 2008, a working group of the British Academy of Medical Sciences noted that many of the trials of piracetam for dementia were flawed.[7][8]
Depression and anxiety
Some sources suggest that piracetam's overall effect on lowering depression and anxiety is higher than on improving memory.[9] However, depression is reported to be an occasional adverse effect of piracetam.[10]
Other
Peripheral vascular effects of piracetam have suggested its use potential for vertigo, dyslexia and sickle cell anemia.[6] A subsequent Cochrane review of the evidence did not support piracetam's use in sickle cell crisis prevention.[11] Piracetam may have potential for the treatment of the symptoms of childhood autism.[12]
Side effects
Piracetam has been found to have very few side effects, and those it has are typically "few, mild, and transient."[13] A large-scale, 12-week trial of high-dose piracetam found no adverse effects occurred in the group taking piracetam as compared to the placebo group.[14] Many other studies have likewise found piracetam to be well tolerated.[13][15][16]
Symptoms of general excitability, including anxiety, insomnia, irritability, headache, agitation, nervousness, tremor, and hyperkinesia, are occasionally reported.[10][17][18] Other reported side effects include somnolence, weight gain, clinical depression, weakness, increased libido, and hypersexuality.[10]
Toxicity
The LD50 for oral consumption in humans has not been determined;[19] however, the LD50 is 5.6 g/kg for rats and 20 g/kg for mice, indicating extremely low acute toxicity.[20]
Mechanisms of action
Piracetam's mechanism of action, as with racetams in general, is not fully understood. The drug influences neuronal and vascular functions and influences cognitive function without acting as a sedative or stimulant.[6] Piracetam is a positive allosteric modulator of the AMPA receptor.[21] It is hypothesized to act on ion channels or ion carriers, thus leading to increased neuron excitability.[19] GABA brain metabolism and GABA receptors are not affected by piracetam [22]
It has been found to increase blood flow and oxygen consumption in parts of the brain, but this may be a side effect of increased brain activity rather than a primary effect or mechanism of action for the drug.[23]
Piracetam improves the function of the neurotransmitter acetylcholine via muscarinic cholinergic (ACh) receptors, which are implicated in memory processes.[24] Furthermore, piracetam may have an effect on NMDA glutamate receptors, which are involved with learning and memory processes. Piracetam is thought to increase cell membrane permeability.[24][25] Piracetam may exert its global effect on brain neurotransmission via modulation of ion channels (i.e., Na+, K+).[19] It has been found to increase oxygen consumption in the brain, apparently in connection to ATP metabolism, and increases the activity of adenylate kinase in rat brains.[26][27] Piracetam, while in the brain, appears to increase the synthesis of cytochrome b5,[28] which is a part of the electron transport mechanism in mitochondria. But in the brain, it also increases the permeability of the mitochondria of some intermediaries of the Krebs cycle.[26]
History
Piracetam was first made in some time between the 1950s and 1964.[29] There are reports of it being used for epilepsy in the 1950s.[30]
Approval
Piracetam is primarily used in Europe, Asia, and South America. In the United States, it is not approved by the US Food and Drug Administration for any medical use and it is not permitted to be sold as a dietary supplement. Piracetam is legal to import into the United Kingdom for personal use with or without prescription. Piracetam has no DIN in Canada, and thus cannot be sold but can be imported for personal use in Canada.[31] It has become popular as a cognitive enhancement drug among students.[32]
Availability

Piracetam is sold under a wide variety of brand names worldwide. Popular trade names for piracetam in Europe are Nootropil and Lucetam, among many others. In Argentina, it is made by GlaxoSmithKline S.A. laboratories and sold under the trade name of Noostan (800 mg or 1200 mg). In Venezuela and Ecuador, piracetam is produced by Laboratorios Farma S.A. and sold under the brand name Breinox. In Mexico it is produced by UCB de Mexico, and sold under the brand name of Nootropil. Other names include Nootropil in the United States, Europe, Brazil, Hong Kong, India, and Mexico; Lucetam, Oikamid, Smart, Geratam, and Biotropil in Europe and Brazil; Neurobasal in Colombia; Breinox in Ecuador and Venezuela;Stimulan in Egypt; and Nocetan in Latin America.
See also
- Aniracetam
- Brivaracetam — an analogue of piracetam with the same additional side chain as levetiracetam and a three–carbon chain. It exhibits greater antiepileptic properties than levetiracetam in animal models, but with a somewhat smaller, although still high, therapeutic range.
- Hydergine
- Levetiracetam — an analogue of piracetam bearing an additional CH3–CH2– sidechain and bearing antiepileptic pharmacological properties through a poorly understood mechanism probably related to its affinity for the vesicle protein SV2A.
- Oxiracetam
- Phenylpiracetam — a phenylated analog of the drug piracetam which was developed in 1983 in Russia where it is available as a prescription drug.
- Pramiracetam
Notes
- ↑ Inspections, Compliance, Enforcement, and Criminal Investigations
- ↑ Enforcement Report - Week of 20 March 2013
- ↑ "Nootropil". NetDoctor.co.uk. 8 July 2004. Retrieved 21 September 2009.
- ↑ Flicker, L; Grimley Evans, G (2001). "Piracetam for dementia or cognitive impairment.". The Cochrane database of systematic reviews (2): CD001011. doi:10.1002/14651858.CD001011. PMID 11405971.
- ↑ Waegemans, T; Wilsher, CR; Danniau, A; Ferris, SH; Kurz, A; Winblad, B (2002). "Clinical efficacy of piracetam in cognitive impairment: a meta-analysis.". Dementia and geriatric cognitive disorders. 13 (4): 217–24. doi:10.1159/000057700. PMID 12006732.
- 1 2 3 Winblad, B (2005). "Piracetam: a review of pharmacological properties and clinical uses". CNS Drug Reviews. 11 (2): 169–82. doi:10.1111/j.1527-3458.2005.tb00268.x. PMID 16007238.
- ↑ Horne G, et al. (May 2008). Brain science, addiction and drugs (PDF) (Report). Academy of Medical Sciences. p. 145. ISBN 1-903401-18-6.
- ↑ Talbot, Margaret (27 April 2009). "Brain Gain: The underground world of 'neuroenhancing' drugs". The New Yorker. Retrieved 21 September 2009.
- ↑ Malykh AG, Sadaie MR (February 2010). "Piracetam and piracetam-like drugs: from basic science to novel clinical applications to CNS disorders". Drugs. 70 (3): 287–312. doi:10.2165/11319230-000000000-00000. PMID 20166767.
- 1 2 3 Nootropil®. Arzneimittel-Kompendium der Schweiz. 2013-09-12. Retrieved 2013-10-27.
- ↑ Al Hajeri, AA; Fedorowicz, Z; Omran, A; Tadmouri, GO (18 April 2007). "Piracetam for reducing the incidence of painful sickle cell disease crises.". The Cochrane database of systematic reviews (2): CD006111. doi:10.1002/14651858.CD006111.pub2. PMID 17443614.
- ↑ Akhondzadeh, S., et al. A double-blind placebo controlled trial of piracetam added to risperidone in patients with autistic disorder. Child Psychiatry and Human Development, Vol 39(3), Sep, 2008. pp. 237–245.
- 1 2 Koskiniemi, M; Van Vleymen, B; Hakamies, L; Lamusuo, S; Taalas, J (1998). "Piracetam relieves symptoms in progressive myoclonus epilepsy: a multicentre, randomised, double blind, crossover study comparing the efficacy and safety of three dosages of oral piracetam with placebo". Journal of neurology, neurosurgery, and psychiatry. 64 (3): 344–8. doi:10.1136/jnnp.64.3.344. PMC 2169975
 . PMID 9527146.
. PMID 9527146. - ↑ De Reuck, J; Van Vleymen, B (1999). "The clinical safety of high-dose piracetam--its use in the treatment of acute stroke". Pharmacopsychiatry. 32 Suppl 1: 33–7. doi:10.1055/s-2007-979234. PMID 10338106.
- ↑ Fedi, M; Reutens, D; Dubeau, F; Andermann, E; D'agostino, D; Andermann, F (2001). "Long-term efficacy and safety of piracetam in the treatment of progressive myoclonus epilepsy". Archives of neurology. 58 (5): 781–6. doi:10.1001/archneur.58.5.781. PMID 11346373.
- ↑ Giurgea, C.; Salama, M. (1977). "Nootropic drugs". Prog Neuropsychopharmacol. 1 (3–4): 235–247. doi:10.1016/0364-7722(77)90046-7.
- ↑ Chouinard, G; Annable, L; Ross-Chouinard, A; Olivier, M; Fontaine, F (1983). "Piracetam in elderly psychiatric patients with mild diffuse cerebral impairment". Psychopharmacology. 81 (2): 100–6. doi:10.1007/BF00429000. PMID 6415738.
- ↑ Hakkarainen, H; Hakamies, L (1978). "Piracetam in the treatment of post-concussional syndrome. A double-blind study". European neurology. 17 (1): 50–5. doi:10.1159/000114922. PMID 342247.
- 1 2 3 Gouliaev, AH; Senning, A (1994). "Piracetam and other structurally related nootropics". Brain research. Brain research reviews. 19 (2): 180–222. doi:10.1016/0165-0173(94)90011-6. PMID 8061686.
- ↑ "Piracetam Material Safety Sheet" (PDF). Spectrum.
- ↑ Ahmed, A; Oswald, R (2010). "Piracetam Defines a New Binding Site for Allosteric Modulators of α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptors". Journal of Medicinal Chemistry. 53 (5): 2197–2203. doi:10.1021/jm901905j. PMC 2872987
 . PMID 20163115.
. PMID 20163115. - ↑ Giurgea, Corneliu E. (1982-01-01). "The nootropic concept and its prospective implications". Drug Development Research. 2 (5): 441–446. doi:10.1002/ddr.430020505. ISSN 1098-2299.
- ↑ Jordaan, B; Oliver, DW; Dormehl, IC; Hugo, N (1996). "Cerebral blood flow effects of piracetam, pentifylline, and nicotinic acid in the baboon model compared with the known effect of acetazolamide". Arzneimittel-Forschung. 46 (9): 844–7. PMID 8876930.
- 1 2 Winnicka, K; Tomasiak, M; Bielawska, A (2005). "Piracetam--an old drug with novel properties?". Acta poloniae pharmaceutica. 62 (5): 405–9. PMID 16459490.
- ↑ Müller, WE; Eckert, GP; Eckert, A (1999). "Piracetam: novelty in a unique mode of action". Pharmacopsychiatry. 32 Suppl 1: 2–9. doi:10.1055/s-2007-979230. PMID 10338102.
- 1 2 Grau, M; Montero, JL; Balasch, J (1987). "Effect of Piracetam on electrocorticogram and local cerebral glucose utilization in the rat". General pharmacology. 18 (2): 205–11. doi:10.1016/0306-3623(87)90252-7. PMID 3569848.
- ↑ Nickolson, VJ; Wolthuis, OL (1976). "Effect of the acquisition-enhancing drug piracetam on rat cerebral energy metabolism. Comparison with naftidrofuryl and methamphetamine". Biochemical pharmacology. 25 (20): 2241–4. doi:10.1016/0006-2952(76)90004-6. PMID 985556.
- ↑ Tacconi, MT; Wurtman, RJ (1986). "Piracetam: physiological disposition and mechanism of action". Advances in neurology. 43: 675–85. PMID 3946121.
- ↑ Li, Jie Jack; Corey, E. J. (2013). Drug Discovery: Practices, Processes, and Perspectives. John Wiley & Sons. p. 276. ISBN 9781118354469.
- ↑ M.D, Emeritus Profess of Neurology and Head of Epilepsy Research Group Berlin Dieter Schmidt; Shorvon, Emeritus Profess of Neurology and Consultant Neurologist Simon (2016). The End of Epilepsy?: A History of the Modern Era of Epilepsy Research 1860-2010. Oxford University Press. p. 69. ISBN 9780198725909.
- ↑ #2, Government of Canada, Health Canada, Health Products and Food Branch, HPFB Inspectorate, Inspectorate Ottawa, Compliance, Enforcement and Coordination Division. "Guidance Document on the Import Requirements for Health Products under the Food and Drugs Act and its Regulations (GUI-0084) [Health Canada, 2010]". www.hc-sc.gc.ca. Retrieved 2016-03-06.
- ↑ Medew, Julia (1 October 2009). "Call for testing on 'smart drugs'". Fairfax Media. Retrieved 29 May 2014.
References
- UCB Pharma Limited (2005). "Nootropil 800 mg & 1200 mg Tablets and Solution". electronic Medicines Compendium. Datapharm Communications. Retrieved 8 December 2005.
External links
| Wikimedia Commons has media related to Piracetam. |