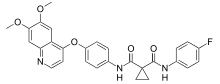
Cabozantinib
 | |
| Clinical data | |
|---|---|
| Trade names | Cabometyx, Cometriq |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | L01XE26 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | ≥99.7% |
| Metabolism | Hepatic (CYP3A4-mediated) |
| Biological half-life | 55 hours |
| Excretion | Faeces (54%), urine (27%) |
| Identifiers | |
| |
| Synonyms | XL184, BMS907351 |
| CAS Number | 849217-68-1 |
| ChemSpider | 25948202 |
| UNII | 1C39JW444G |
| KEGG | D10062 |
| ChEBI |
CHEBI:72317 |
| ChEMBL | CHEMBL2105717 |
| ECHA InfoCard | 100.221.147 |
| Chemical and physical data | |
| Formula | C28H24FN3O5 |
| Molar mass | 501.51 g mol |
| 3D model (Jmol) | Interactive image |
| |
| |
Cabozantinib, marketed under the trade name Cabometyx among others, is a small molecule inhibitor of the tyrosine kinases c-Met and VEGFR2, and has been shown to reduce tumor growth, metastasis, and angiogenesis. It was discovered and developed by Exelixis Inc.
Cabozantinib was granted orphan drug status by the U.S. Food and Drug Administration (FDA) in January 2011.[1] Cabozantinib is approved by the U.S. FDA for medullary thyroid cancer.[2] and advanced renal cell carcinoma in people who have received prior anti-angiogenic therapy.[3] It is currently undergoing clinical trials for the treatment of prostate, bladder, ovarian, brain, melanoma, breast, non-small cell lung, pancreatic, and hepatocellular cancers.
Cabozantinib will be distributed in Europe by the French pharmaceutical company Ipsen after a collaboration was reached with Exelixis in March 2016.[4]
Approvals and indications
In October 2011, cabozantinib met its primary endpoint in a phase 3 clinical trial (EXAM) conducted by Exelixis investigating its effect on progression-free survival in medullary thyroid cancer.[5] A new drug application was submitted in the first half of 2012,[6] and on November 29, 2012 cabozantinib in its capsule formulation was granted marketing approval by the U.S. FDA under the name Cometriq for treating patients with medullary thyroid cancer.[2]
Approval for its tablet formulation was granted for treating people with kidney cancer on April 25th, 2016.[7]
Grapefruit and grapefruit juice should be avoided as they may increase the concentration of the drug in the blood.[8] It is not yet known if cabozantinib is safe and effective in children.
Clinical trials
It is undergoing clinical trials for the treatment of prostate, ovarian, brain, melanoma, breast, non-small cell lung, hepatocellular and kidney cancers.[9]
Kidney cancer
A phase 3 study of cabozantinib versus everolimus in people with advanced clear renal cell carcinoma that worsened after VEGFR-target therapy, found benefit with cabozantinib.[10]
In a November 2015 vote, the National Comprehensive Cancer Network granted cabozantinib category 1 designation in advanced renal cell carcinoma for people who have received one prior VEGFR targeted tyrosine kinase inhibitor. [11] On April 25th 2016, the FDA granted approval for cabozantinib for second-line treatment of advanced clear renal cell carcinoma. [12]
Glioblastoma multiforme
In 2009 a phase II study for relapsed glioblastoma multiforme reported encouraging interim results.[13]
Prostate cancer
Positive data from clinical trials in 2011 indicate cabozantinib is beneficial in metastatic advanced prostate cancer (castration-resistant prostate cancer). 97% of patients either had stabilization or improvement in bone malignancies. The median time to disease progression was 29 weeks.[14][15]
One US trial reported in May 2011: The best results were seen in patients with liver, prostate, and ovarian cancer: 22 of 29 patients with liver cancer, 71 of 100 patients with prostate cancer, and 32 of 51 with ovarian cancer experienced either partial tumor shrinkage or stable disease. Fifty-nine out of 68 patients who had bone metastases had their metastases shrink or disappear during the trial.[16]
See also
References
- ↑ Exelixis’ XL184 Granted Orphan Drug Designation and Assigned the Generic Name Cabozantinib. Jan 2011
- 1 2 "FDA approves Cometriq to treat rare type of thyroid cancer". 29 November 2012.
- ↑ FDA Approval Announcement, April 2016
- ↑ http://www.exelixis.com/investors-media/press-releases?cpurl=http%3A%2F%2Fir.exelixis.com/phoenix.zhtml?c=120923%26p=irol-newsArticle%26ID=2144302%26highlight=
- ↑ "Success for the EXAM trial". Retrieved 24 October 2011.
- ↑ "Thyroid cancer drug cabozantinib prolongs PFS". Retrieved 24 October 2011.
- ↑ http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208692s000lbl.pdf
- ↑ "Cometriq prescribing information" (PDF). Retrieved 29 November 2012.
- ↑ "Cabozantinib - List Results - ClinicalTrials.gov". U.S. National Institute of Health. Retrieved 25 April 2013.
- ↑ Choueiri, TK; Escudier, B; Powles, T; Mainwaring, PN; Rini, BI; Donskov, F; Hammers, H; Hutson, TE; Lee, JL; Peltola, K; Roth, BJ; Bjarnason, GA; Géczi, L; Keam, B; Maroto, P; Heng, DY; Schmidinger, M; Kantoff, PW; Borgman-Hagey, A; Hessel, C; Scheffold, C; Schwab, GM; Tannir, NM; Motzer, RJ; METEOR, Investigators (5 November 2015). "Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma.". The New England Journal of Medicine. 373 (19): 1814–23. doi:10.1056/nejmoa1510016. PMID 26406150.
- ↑ http://www.nccn.org/disclosures/MinuteHandler.ashx?fileManagerId=280
- ↑ http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Overview&DrugName=CABOMETYX
- ↑ "A phase II study of XL184 in patients (pts) with progressive glioblastoma multiforme (GBM) in first or second relapse.". 2009.
- ↑ "Exelixis drug slows prostate cancer spread in trial". Reuters. 6 June 2011.
- ↑ Cabozantinib (XL184) Phase 2 Data Demonstrate Encouraging Clinical Activity in Patients with Castration-Resistant Prostate Cancer. Feb 2011
- ↑ "Cabozantinib Shrinks Tumors and Bone Metastases in Prostate and Other Cancers". 31 May 2011.
External links
- Exelixis: Looming Cancer Conference a Favorable Swing Trade Opportunity
- A phase II study of XL184 in patients (pts) with progressive glioblastoma multiforme (GBM) in first or second relapse. 2009