Toxoplasmosis
| Toxoplasmosis | |
|---|---|
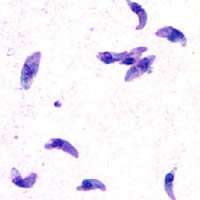 | |
| T. gondii tachyzoites | |
| Classification and external resources | |
| Specialty | Infectious disease |
| ICD-10 | B58 |
| ICD-9-CM | 130 |
| DiseasesDB | 13208 |
| MedlinePlus | 000637 |
| eMedicine | med/2294 |
| Patient UK | Toxoplasmosis |
| MeSH | D014123 |
Toxoplasmosis is a parasitic disease caused by Toxoplasma gondii.[1] Infections with toxoplasmosis usually cause no symptoms in adult humans.[2] Occasionally there may be a few weeks or months of mild flu-like illness such as muscle aches and tender lymph nodes.[3] In a small number of people, eye problems may develop. In those with a weak immune system, severe symptoms such as seizures and poor coordination may occur. If infected during pregnancy, a condition known as congenital toxoplasmosis may affect the child.[3]
Toxoplasmosis is usually spread by eating poorly cooked food that contains cysts, exposure to infected cat feces, and from a mother to a child during pregnancy if the mother becomes infected. Rarely the disease may be spread by a blood transfusion. It is not otherwise spread between people.[1] The parasite is only known to reproduce sexually in the cat family. However, it can infect most types of warm-blooded animals, including humans.[4] Diagnosis is typically by testing the blood for antibodies or by testing the amniotic fluid for the parasite's DNA.[5]
Prevention is by properly preparing and cooking food. It is also recommended that pregnant women not clean cat litter boxes.[6] Treatment of otherwise healthy people is usually not needed. During pregnancy spiramycin or pyrimethamine/sulfadiazine and folinic acid may be used for treatment.[7]
Up to half of the world's population is infected by toxoplasmosis but have no symptoms.[8] In the United States about 23% are affected[9] and in some areas of the world this is up to 95%.[1] About 200,000 cases of congenital toxoplasmosis occur a year.[10] Charles Nicolle and Louis Manceaux first described the organism in 1908. In 1941 transmission during pregnancy from a mother to a child was confirmed.[11]
Signs and symptoms
Infection has three stages:
Acute toxoplasmosis
Acute toxoplasmosis is often asymptomatic in healthy adults.[12][13] However, symptoms may manifest and are often influenza-like: swollen lymph nodes, headaches, fever, and fatigue,[14] or muscle aches and pains that last for a month or more. Rarely will a human with a fully functioning immune system develop severe symptoms following infection. People with weakened immune systems are likely to experience headache, confusion, poor coordination, seizures, lung problems that may resemble tuberculosis or Pneumocystis jiroveci pneumonia (a common opportunistic infection that occurs in people with AIDS), or blurred vision caused by severe inflammation of the retina (ocular toxoplasmosis)[14] Young children and immunocompromised people, such as those with HIV/AIDS, those taking certain types of chemotherapy, or those who have recently received an organ transplant, may develop severe toxoplasmosis. This can cause damage to the brain (encephalitis) or the eyes (necrotizing retinochoroiditis).[15] Infants infected via placental transmission may be born with either of these problems, or with nasal malformations, although these complications are rare in newborns. The toxoplasmic trophozoites causing acute toxoplasmosis are referred to as tachyzoites, and are typically found in bodily fluids.
Swollen lymph nodes are commonly found in the neck or under the chin, followed by the armpits and the groin. Swelling may occur at different times after the initial infection, persist, and recur for various times independently of antiparasitic treatment.[16] It is usually found at single sites in adults, but in children, multiple sites may be more common. Enlarged lymph nodes will resolve within one to two months in 60% of cases. However, a quarter of those affected take two to four months to return to normal, and 8% take four to six months. A substantial number (6%) do not return to normal until much later.[17]
Latent toxoplasmosis
Due to its asymptomatic nature,[12][13] it is easy for a host to become infected with Toxoplasma gondii and develop toxoplasmosis without knowing it. Although mild, flu-like symptoms occasionally occur during the first few weeks following exposure, infection with T. gondii produces no readily observable symptoms in healthy human adults.[8][18] In most immunocompetent people, the infection enters a latent phase, during which only bradyzoites (tissue cysts) are present;[19] these tissue cysts and even lesions can occur in the retinas, alveolar lining of the lungs (where an acute infection may mimic a Pneumocystis jirovecii infection), heart, skeletal muscle, and the central nervous system (CNS), including the brain.[20] Cysts form in the CNS (brain tissue) upon infection with T. gondii and persist for the lifetime of the host.[21] Most infants who are infected while in the womb have no symptoms at birth, but may develop symptoms later in life.[22]
Reviews of serological studies have estimated that 30–50% of the global population has been exposed to and may be chronically infected with latent toxoplasmosis, although infection rates differ significantly from country to country.[8][23][24] This latent state of infection has recently been associated with numerous disease burdens,[8] neural alterations,[21][23] and subtle gender-dependent behavioral changes in immunocompetent humans.[25][26]
Cutaneous toxoplasmosis
While rare, skin lesions may occur in the acquired form of the disease, including roseola and erythema multiforme-like eruptions, prurigo-like nodules, urticaria, and maculopapular lesions. Newborns may have punctate macules, ecchymoses, or “blueberry muffin” lesions. Diagnosis of cutaneous toxoplasmosis is based on the tachyzoite form of T. gondii being found in the epidermis.[27] It is found in all levels of the epidermis, is about 6 μm by 2 μm and bow-shaped, with the nucleus being one-third of its size. It can be identified by electron microscopy or by Giemsa staining tissue where the cytoplasm shows blue, the nucleus red.[28]
Cause

Parasitology
In its life cycle, T. gondii adopts several forms.[29] Tachyzoites are responsible for acute infection; they divide rapidly and spread through the tissues of the body. After proliferating, tachyzoites convert into bradyzoites, which take the form of latent intracellular tissue cysts that form mainly in the tissues of the muscles and brain. The transformation into cysts is in part triggered by the pressure of the host immune system.[30] The bradyzoites are not responsive to antibiotics. The bradyzoites, once formed, can remain in the tissues for the lifespan of the host. In a healthy host, if some bradyzoites convert back into active tachyzoites, the immune system will quickly destroy them. However, in immunocompromised individuals, or in fetuses, which lack a developed immune system, the tachyzoites can run rampant and cause significant neurological damage.[29]
The parasite’s survival is dependent on a balance between host survival and parasite proliferation.[30] T. gondii achieves this balance by manipulating the host’s immune response, reducing the host’s immune response and enhancing the parasite’s reproductive advantage.[30] Once it infects a normal host cell, it resists damage caused by the host’s immune system, and changes the host's immune processes.
As it forces its way into the host cell, the parasite forms a parasitophorous vacuole (PV) membrane from the membrane of the host cell.[2][31] The PV encapsulates the parasite, and is both resistant to the activity of the endolysosomal system, and can take control of the host’s mitochondria and endoplasmic reticulum.[2][31]
When first invading the cell, the parasite releases ROP proteins from the bulb of the rhoptry organelle.[2] These proteins translocate to the nucleus and the surface of the PV membrane where they can activate STAT pathways to modulate the expression of cytokines at the transcriptional level, bind and inactivate PV membrane destroying IRG proteins, among other possible effects.[2][31][32] Additionally, certain strains of T. gondii can secrete a protein known as GRA15, activating the NF-κB pathway, which upregulates the pro-inflammatory cytokine IL-12 in the early immune response, possibly leading to the parasite’s latent phase.[2] The parasite’s ability to secrete these proteins depends on its genotype and affects its virulence.[2][32]
The parasite also influences an anti-apoptotic mechanism, allowing the infected host cells to persist and replicate. One method of apoptosis resistance is by disrupting pro-apoptosis effector proteins, such as BAX and BAK.[33] To disrupt these proteins, T. gondii causes conformational changes to the proteins, which prevent the proteins from being transported to various cellular compartments where they initiate apoptosis events. T. gondii does not, however, cause downregulation of the pro-apoptosis effector proteins.[33]
T. gondii also has the ability to initiate autophagy of the host’s cells.[34] This leads to a decrease in healthy, uninfected cells, and consequently fewer host cells to attack the infected cells. Research by Wang et al finds that infected cells lead to higher levels of autophagosomes in normal and infected cells.[34] Their research reveals that T. gondii causes host cell autophagy using a calcium-dependent pathway.[34] Another study suggests that the parasite can directly affect calcium being released from calcium stores, which are important for the signalling processes of cells.[33]
The mechanisms above allow T. gondii to persist in a host. Some limiting factors for the toxoplasma is that its influence on the host cells is stronger in a weak immune system and is quantity-dependent, so a large number of T. gondii per host cell cause a more severe effect.[35] The effect on the host also depends on the strength of the host immune system. Immunocompetent individuals do not normally show severe symptoms or any at all, while fatality or severe complications can result in immunocompromised individuals.[35]
It should be noted that since the parasite can change the host’s immune response, it may also have an effect, positive or negative, on the immune response to other pathogenic threats.[30] This includes, but is not limited to, the responses to infections by Helicobacter felis, Leishmania major, or other parasites, such as Nippostrongylus brasiliensis.[30]
Transmission
Toxoplasmosis is generally transmitted through the mouth when toxoplama gondii cysts are accidentally eaten.[36] Congenital transmittance from the mother to fetus can also occur.[37]
Oral transmission may occur through:
- Ingestion of raw or partly cooked meat, especially pork, lamb, or venison containing Toxoplasma cysts: Infection prevalence in countries where undercooked meat is traditionally eaten has been related to this transmission method. Tissue cysts may also be ingested during hand-to-mouth contact after handling undercooked meat, or from using knives, utensils, or cutting boards contaminated by raw meat.[38]
- Ingestion of unwashed fruits or vegetables that have been in contact with contaminated soil containing infected cat feces.[39]
- Ingestion of contaminated cat feces: This can occur through hand-to-mouth contact following gardening, cleaning a cat's litter box, contact with children's sandpits; the parasite can survive in the environment for over a year.[40]
Cats excrete the pathogen in their feces for a number of weeks after contracting the disease, generally by eating an infected rodent. Even then, cat feces are not generally contagious for the first day or two after excretion, after which the cyst 'ripens' and becomes potentially pathogenic.[41] In addition to cats, birds and mammals including human beings are also intermediate host of the spores and are involved in the transmission process. However the pathogenicity varies with the age and species involved in infection and the mode of transmission of T. gondii.[42]
Pregnancy precautions
Congenital toxoplasmosis is a specific form of toxoplasmosis in which an unborn fetus is infected via the placenta.[43] Congenital toxoplasmosis is associated with fetal death and abortion, and in infants, it is associated with neurologic deficits, neurocognitive deficits, and chorioretinitis.[44] A positive antibody titer indicates previous exposure and immunity, and largely ensures the unborn fetus' safety. A simple blood draw at the first prenatal doctor visit can determine whether or not a woman has had previous exposure and therefore whether or not she is at risk. If a woman receives her first exposure to T. gondii while pregnant, the fetus is at particular risk.[44]
Not much evidence exists around the effect of education before pregnancy to prevent congenital toxoplasmosis.[45] However educating parents before the baby is born has been suggested to be effective because it may improve food, personal and pet hygiene.[45] More research is needed to find whether antenatal education can reduce congenital toxoplasmosis.[45]
For pregnant women with negative antibody titers, indicating no previous exposure to T. gondii, serology testing as frequent as monthly is advisable as treatment during pregnancy for those women exposed to T. gondii for the first time dramatically decreases the risk of passing the parasite to the fetus. Since a baby's immune system does not develop fully for the first year of life, and the resilient cysts that form throughout the body are very difficult to eradicate with antiprotozoans, an infection can be very serious in the young.
Despite these risks, pregnant women are not routinely screened for toxoplasmosis in most countries, for reasons of cost-effectiveness and the high number of false positives generated; Portugal,[46] France,[47] Austria,[47] Uruguay,[48] and Italy[49] are notable exceptions, and some regional screening programmes operate in Germany, Switzerland and Belgium.[49] As invasive prenatal testing incurs some risk to the fetus (18.5 pregnancy losses per toxoplasmosis case prevented),[47] postnatal or neonatal screening is preferred. The exceptions are cases where fetal abnormalities are noted, and thus screening can be targeted.[47]
Pregnant women should avoid handling raw meat, drinking raw milk (especially goat milk) and be advised to not eat raw or undercooked meat regardless of type.[50] Because of the obvious relationship between Toxoplasma and cats it is also often advised to avoid exposure to cat feces, and refrain from gardening (cat feces are common in garden soil) or at least wear gloves when so engaged.[50] Most cats are not actively shedding oocysts, since they get infected in the first six months of their life, when they shed oocysts for a short period of time (1–2 weeks.)[51] However, these oocysts get buried in the soil, sporulate and remain infectious for periods ranging from several months to more than a year.[50] Numerous studies have shown living in a household with a cat is not a significant risk factor for T. gondii infection,[50][52][53] though living with several kittens has some significance.[54]
In 2006, a Czech research team[55] discovered women with high levels of toxoplasmosis antibodies were significantly more likely to have baby boys than baby girls. In most populations, the birth rate is around 51% boys, but women infected with T. gondii had up to a 72% chance of a boy.[56][57] In mice, the sex ratio was higher in early latent toxoplasmosis and lower in later latent toxoplasmosis.[57]
Rodent behavior
Infection with T. gondii has been shown to alter the behavior of mice and rats in ways thought to increase the rodents’ chances of being preyed upon by cats.[58][59][60] Infected rodents show a reduction in their innate aversion to cat odors; while uninfected mice and rats will generally avoid areas marked with cat urine or with cat body odor, this avoidance is reduced or eliminated in infected animals.[58][60][61] Moreover, some evidence suggests this loss of aversion may be specific to feline odors: when given a choice between two predator odors (cat or mink), infected rodents show a significantly stronger preference to cat odors than do uninfected controls.[62][63]
In rodents, T. gondii–induced behavioral changes occur through epigenetic remodeling in neurons associated with observed behaviors;[64][65] for example, it modifies epigenetic methylation to induce hypomethylation of arginine vasopressin-related genes in the medial amygdala to greatly decrease predator aversion.[64][65] Similar epigenetically-induced behavioral changes have also been observed in mouse models of addiction, where changes in the expression of histone-modifying enzymes via gene knockout or enzyme inhibition in specific neurons produced alterations in drug-related behaviors.[66][67][68] Widespread histone-lysine acetylation in cortical astrocytes appears to be another epigenetic mechanism employed by T. gondii.[69][70]
T. gondii-infected rodents show a number of behavioral changes beyond altered responses to cat odors. Rats infected with the parasite show increased levels of activity and decreased neophobic behavior.[71] Similarly, infected mice show alterations in patterns of locomotion and exploratory behavior during experimental tests. These patterns include traveling greater distances, moving at higher speeds, accelerating for longer periods of time, and showing a decreased pause-time when placed in new arenas.[72] Infected rodents have also been shown to have lower anxiety, using traditional models such as elevated plus mazes, open field arenas, and social interaction tests.[72][73]
Diagnosis
Diagnosis of toxoplasmosis in humans is made by biological, serological, histological, or molecular methods, or by some combination of the above.[51] Toxoplasmosis can be difficult to distinguish from primary central nervous system lymphoma. It mimics several other infectious diseases so clinical signs are non-specific and are not sufficiently characteristic for a definite diagnosis. As a result, the diagnosis is made by a trial of therapy (pyrimethamine, sulfadiazine, and folinic acid (USAN: leucovorin)), if the drugs produce no effect clinically and no improvement on repeat imaging.
T. gondii may also be detected in blood, amniotic fluid, or cerebrospinal fluid by using polymerase chain reaction.[74] T. gondii may exist in a host as an inactive cyst that would likely evade detection.
Serological testing can detect T. gondii antibodies in the blood serum, using methods including the Sabin–Feldman dye test (DT), the indirect hemagglutination assay, the indirect fluorescent antibody assay (IFA), the direct agglutination test, the latex agglutination test (LAT), the enzyme-linked immunosorbent assay (ELISA), and the immunosorbent agglutination assay test (IAAT).[51]
The most commonly used tests to measure IgG antibody are the DT, the ELISA, the IFA, and the modified direct agglutination test.[75] IgG antibodies usually appear within a week or two of infection, peak within one to two months, then decline at various rates.[75] Toxoplasma IgG antibodies generally persist for life, and therefore may be present in the bloodstream as a result of either current or previous infection.[9]
To some extent, acute toxoplasmosis infections can be differentiated from chronic infections using an IgG avidity test, which is a variation on the ELISA. In the first response to infection, toxoplasma-specific IgG has a low affinity for the toxoplasma antigen; in the following weeks and month, IgG affinity for the antigen increases. Based on the IgG avidity test, if the IgG in the infected individual has a high affinity, it means that the infection began three to five months before testing. This is particularly useful in congenital infection, where pregnancy status and gestational age at time of infection determines treatment.[76]
In contrast to IgG, IgM antibodies can be used to detect acute infection, but generally not chronic infection.[9] The IgM antibodies appear sooner after infection than the IgG antibodies and disappear faster than IgG antibodies after recovery.[51] In most cases, T. gondii-specific IgM antibodies can first be detected approximately a week after acquiring primary infection, and decrease within one to six months; 25% of those infected are negative for T. gondii-specific IgM within seven months.[9] However, IgM may be detectable months or years after infection, during the chronic phase, and false positives for acute infection are possible.[75] The most commonly used tests for the measurement of IgM antibody are double-sandwich IgM-ELISA, the IFA test, and the immunosorbent agglutination assay (IgM-ISAGA). Commercial test kits often have low specificity, and the reported results are frequently misinterpreted.[75]
Congenital toxoplasmosis
Recommendations for the diagnosis of congenital toxoplasmosis include: prenatal diagnosis based on testing of amniotic fluid and ultrasound examinations; neonatal diagnosis based on molecular testing of placenta and cord blood and comparative mother-child serologic tests and a clinical examination at birth; and early childhood diagnosis based on neurologic and ophthalmologic examinations and a serologic survey during the first year of life.[43] During pregnancy, serological testing is recommended at three week intervals.[77]
Even though diagnosis of toxoplasmosis heavily relies on serological detection of specific anti-Toxoplasma immunoglobulin, serological testing has limitations. For example, it may fail to detect the active phase of T. gondii infection because the specific anti-Toxoplasma IgG or IgM may not be produced until after several weeks of infection. As a result, a pregnant woman might test negative during the active phase of T. gondii infection leading to undetected and therefore untreated congenital toxoplasmosis.[78] Also, the test may not detect T. gondii infections in immunocompromised patients because the titers of specific anti-Toxoplasma IgG or IgM may not rise in this type of patient.
Many PCR-based techniques have been developed to diagnose toxoplasmosis using clinical specimens that include amniotic fluid, blood, cerebrospinal fluid, and tissue biopsy. The most sensitive PCR-based technique is nested PCR, followed by hybridization of PCR products.[78] The major downside to these techniques is that they are time consuming and do not provide quantitative data.[78]
Real-time PCR is useful in pathogen detection, gene expression and regulation, and allelic discrimination. This PCR technique utilizes the 5' nuclease activity of Taq DNA polymerase to cleave a nonextendible, fluorescence-labeled hybridization probe during the extension phase of PCR.[78] A second fluorescent dye, e.g., 6-carboxy-tetramethyl-rhodamine, quenches the fluorescence of the intact probe.[78] The nuclease cleavage of the hybridization probe during the PCR releases the effect of quenching resulting in an increase of fluorescence proportional to the amount of PCR product, which can be monitored by a sequence detector.[78]
Toxoplasmosis cannot be detected with immunostaining. Lymph nodes affected by Toxoplasma have characteristic changes, including poorly demarcated reactive germinal centers, clusters of monocytoid B cells, and scattered epithelioid histiocytes.
Treatment
Treatment is often only recommended for people with serious health problems, such as people with HIV whose CD4 counts are under 200 cells/mm3, because the disease is most serious when one's immune system is weak. Trimethoprim/sulfamethoxazole is the drug of choice to prevent toxoplasmosis, but not for treating active disease. A new study (May 2012) shows a promising new way to treat the active and latent form of this disease using two endochin-like quinolones.[79]
Acute
The medications prescribed for acute toxoplasmosis are the following:
- Pyrimethamine — an antimalarial medication
- Sulfadiazine — an antibiotic used in combination with pyrimethamine to treat toxoplasmosis
- Combination therapy is usually given with folic acid supplements to reduce incidence of thrombocytopaenia.
- Combination therapy is most useful in the setting of HIV.
- Clindamycin
- Spiramycin — an antibiotic used most often for pregnant women to prevent the infection of their children.
(other antibiotics, such as minocycline, have seen some use as a salvage therapy).
If infected during pregnancy, spiramycin is recommended in the first and early second trimesters while pyrimethamine/sulfadiazine and leucovorin is recommended in the late second and third trimesters.[80]
Latent
In people with latent toxoplasmosis, the cysts are immune to these treatments, as the antibiotics do not reach the bradyzoites in sufficient concentration.
The medications prescribed for latent toxoplasmosis are:
- Atovaquone — an antibiotic that has been used to kill Toxoplasma cysts inside AIDS patients[81]
- Clindamycin — an antibiotic that, in combination with atovaquone, seemed to optimally kill cysts in mice[82]
Congenital toxoplasmosis
When a pregnant woman is diagnosed with acute toxoplasmosis, amniocentesis can be used to determine whether the fetus has been infected or not. When a pregnant woman develops acute toxoplasmosis, the tachyzoites have approximately a 30% chance of entering the placental tissue, and from there entering and infecting the fetus. As gestational age at the time of infection increases, the chance of fetal infection also increases.[29]
If the parasite has not yet reached the fetus, spiramycin can help to prevent placental transmission. If the fetus has been infected, the pregnant woman can be treated with pyrimethamine and sulfadiazine, with folinic acid, after the first trimester. They are given after the first trimester and with folinic acid because pyrimethamine has an antifolate effect, and lack of folic acid can interfere with fetal brain formation and cause thrombocytopaenia.[83] Infection in earlier gestational stages correlates with poorer fetal and neonatal outcomes, particularly when the infection is untreated.[84]
Epidemiology
T. gondii infections occur throughout the world, although infection rates differ significantly by country.[85] For women of childbearing age, a survey of 99 studies within 44 countries found the areas of highest prevalence are within Latin America (about 50–80%), parts of Eastern and Central Europe (about 20–60%), the Middle East (about 30-50%), parts of Southeast Asia (about 20–60%), and parts of Africa (about 20–55%).[85]
In the United States, data from the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2004 found 9.0% of US-born persons 12–49 years of age were seropositive for IgG antibodies against T. gondii, down from 14.1% as measured in the NHANES 1988–1994.[86] In the 1999–2004 survey, 7.7% of US-born and 28.1% of foreign-born women 15–44 years of age were T. gondii seropositive.[86] A trend of decreasing seroprevalence has been observed by numerous studies in the United States and many European countries.[85]
The protist responsible for Toxoplasmosis is T. gondii. There are three major types of T. gondii responsible for the patterns of Toxoplasmosis throughout the world. There are types I, II, and III. These three types of T. gondii have differing effects on certain hosts, mainly mice and humans due to their variation in genotypes.[57]
- Type I: virulent in mice and humans, seen in AIDS patients.
- Type II: non-virulent in mice, virulent in humans (mostly Europe and North America), seen in AIDS patients.
- Type III: non-virulent in mice, virulent mainly in animals but seen to a lesser degree in humans as well.
Current serotyping techniques can only separate type I or III from type II parasites.[87]
Because the parasite poses a particular threat to fetuses when it is contracted during pregnancy,[88] much of the global epidemiological data regarding T. gondii comes from seropositivity tests in women of childbearing age. Seropositivity tests look for the presence of antibodies against T. gondii in blood, so while seropositivity guarantees one has been exposed to the parasite, it does not necessarily guarantee one is chronically infected.[89]
History
Toxoplasma gondii was first described in 1908 by Nicolle and Manceaux in Tunisia, and independently by Splendore in Brazil.[11] Splendore reported the protozoan in a rabbit, while Nicolle and Manceaux identified it in a North African rodent, the gundi (Ctenodactylus gundi).[90] In 1909 Nicolle and Manceaux differentiated the protozoan from Leishmania.[11] Nicolle and Manceaux then named it Toxoplasma gondii after the curved shape of its infectious stage (Greek root ‘toxon’= bow).[11]
The first recorded case of congenital toxoplasmosis was in 1923, but it was not identified as caused by T. gondii.[90] Janků (1923) described in detail the autopsy results of an 11-month-old boy who had presented to hospital with hydrocephalus. The boy had classic marks of toxoplasmosis including chorioretinitis (inflammation of the choroid and retina of the eye).[90] Histology revealed a number of “sporocytes”, though Janků did not identify these as T. gondii.[90]
It was not until 1937 that the first detailed scientific analysis of T. gondii took place using techniques previously developed for analyzing viruses.[11] In 1937 Sabin and Olitsky analyzed T. gondii in laboratory monkeys and mice. Sabin and Olitsky showed that T. gondii was an obligate intracellular parasite and that mice fed T. gondii-contaminated tissue also contracted the infection.[11] Thus Sabin and Olitsky demonstrated T. gondii as a pathogen transmissible between animals.
T. gondii was first identified as a human pathogen in 1939.[11] Wolf, Cowen and Paige identified T. gondii infection in an infant girl delivered full-term by Caesarean section.[90] The infant developed seizures and had chorioretinitis in both eyes at three days. The infant then developed encephalomyelitis and died at one month of age. Wolf, Cowen and Paige isolated T. gondii from brain tissue lesions. Intracranial injection of brain and spinal cord samples into mice, rabbits and rats produced encephalitis in the animals.[11] Wolf, Cowen and Page reviewed additional cases and concluded that T. gondii produced recognizable symptoms and could be transmitted from mother to child.[90]
The first adult case of toxoplasmosis was reported in 1940 with no neurological signs. Pinkerton and Weinman reported the presence of Toxoplasma in a 22-year-old man from Peru who died from a subsequent bacterial infection and fever.[90]
In 1948, a serological dye test was created by Sabin and Feldman based on the ability of the patient’s antibodies to alter staining of Toxoplasma.[11][91] The Sabin Feldman Dye Test is now the gold standard for identifying Toxoplasma infection.[11]
Transmission of Toxoplasma by eating raw or undercooked meat was demonstrated by Desmonts et al. in 1965 Paris.[11] Desmonts observed that the therapeutic consumption of raw beef or horse meat in a tuberculosis hospital was associated with a 50% per year increase in Toxoplasma antibodies.[11] This means that more T. gondii was being transmitted through the raw meat.
In 1974, Desmonts and Couvreur showed that infection during the first two trimesters produces most harm to the fetus, that transmission depended on when mothers were infected during pregnancy, that mothers with antibodies before pregnancy did not transmit the infection to the fetus, and that spiramycin lowered the transmission to the fetus.[90]
Toxoplasma gained more attention in the 1970s with the rise of immune-suppressant treatment given after organ or bone marrow transplants and the AIDS epidemic of the 1980s.[11] Patients with lowered immune system function are much more susceptible to disease.
Society and culture
"Crazy cat-lady syndrome"
"Crazy cat-lady syndrome" is a term coined by news organizations to describe scientific findings that link the parasite Toxoplasma gondii to several mental disorders and behavioral problems.[92][93][94][95][96] Although researches found that cat ownership does not strongly increase the risk of a T. gondii infection in pregnant women,[97][98] the suspected correlation between cat ownership in childhood and later development of schizophrenia suggest that further studies are needed to determine a risk factor for children.[99] The term crazy cat-lady syndrome draws on both stereotype and popular cultural reference. It was originated as instances of the aforementioned afflictions were noted amongst the populace. Cat lady is a cultural stereotype of a woman, often a spinster, who compulsively hoards and dotes upon cats. Jaroslav Flegr (biologist) is a proponent of the theory that toxoplasmosis affects human behaviour.[100][101]
Notable cases
- Arthur Ashe (tennis player) developed neurological problems from toxoplasmosis (and was later found to be HIV-positive).[102]
- Merritt Butrick (actor) was HIV-positive and died from toxoplasmosis as a result of his already-weakened immune system.[103]
- Pedro Zamora (reality television personality and HIV/AIDS activist) was diagnosed with toxoplasmosis as a result of his immune system being weakened by HIV.[104][105]
- Prince François, Count of Clermont (pretender to the throne of France); his disability caused him to be overlooked in the line of succession.
- Leslie Ash (actress) contracted toxoplasmosis in the second month of pregnancy.[106]
- Sebastian Coe (British middle-distance runner).[107]
- Martina Navratilova suffered from toxoplasmosis during the 1982 US Open.[108]
- Louis Wain (artist) was famous for painting cats; some claim he later developed schizophrenia, which some believe was due to toxoplasmosis resulting from his prolonged exposure to cats.[109]
- The Wealdstone Raider suffered toxoplasmosis for much of his childhood and adolescence.[110]
Popular culture
Literature
- In Irvine Welsh's debut novel Trainspotting (1993), the character Matty dies of toxoplasmosis.[111] In the film adaptation of the novel, it is the HIV-positive character Tommy who dies of toxoplasmosis.
Other animals

Although T. gondii has the capability of infecting virtually all warm-blooded animals, susceptibility and rates of infection vary widely between different genera and species.[114] Rates of infection in populations of the same species can also vary widely due to differences in location, diet, and other factors.
Although infection with T. gondii has been noted in several species of Asian primates, seroprevalence of T. gondii antibodies was found for the first time in toque macaques (Macaca sinica) that are endemic to the island of Sri Lanka.[115]
Livestock
Among livestock, pigs, sheep[116] and goats have the highest rates of chronic T. gondii infection.[117] The prevalence of T. gondii in meat-producing animals varies widely both within and among countries,[117] and rates of infection have been shown to be dramatically influenced by varying farming and management practices.[13] For instance, animals kept outdoors or in free-ranging environments are more at risk of infection than animals raised indoors or in commercial confinement operations.[13][118]
In the United States, the percentage of pigs harboring viable parasites has been measured (via bioassay in mice or cats) to be as high as 92.7% and as low as 0%, depending on the farm or herd.[118] Surveys of seroprevalence (T. gondii antibodies in blood) are more common, and such measurements are indicative of the high relative seroprevalence in pigs across the world.[119] Along with pigs, sheep and goats are among the most commonly infected livestock of epidemiological significance for human infection.[117] Prevalence of viable T. gondii in sheep tissue has been measured (via bioassay) to be as high as 78% in the United States,[120] and a 2011 survey of goats intended for consumption in the United States found a seroprevalence of 53.4%.[121]
Due to a lack of exposure to the outdoors, chickens raised in large-scale indoor confinement operations are not commonly infected with T. gondii.[13] Free-ranging or backyard-raised chickens are much more commonly infected.[13] A survey of free-ranging chickens in the United States found its prevalence to be 17%–100%, depending on the farm.[122] Because chicken meat is generally cooked thoroughly before consumption, poultry is not generally considered to be a significant risk factor for human T. gondii infection.[123]
Although cattle and buffalo can be infected with T. gondii, the parasite is generally eliminated or reduced to undetectable levels within a few weeks following exposure.[13] Tissue cysts are rarely present in buffalo meat or beef, and meat from these animals is considered to be low-risk for harboring viable parasites.[117][118]
Horses are considered resistant to chronic T. gondii infection.[13] However, viable cells have been isolated from US horses slaughtered for export, and severe human toxoplasmosis in France has been epidemiologically linked to the consumption of horse meat.[118][124]
Domestic cats
In 1942, first case of feline toxoplasmosis was diagnosed and reported in a domestic cat in Middletown, NY.[125] The investigators were able to isolate oocysts from feline feces and it was also noted that the oocysts can be infectious for up to 12 months in the environment.[126]
The seroprevalence of T. gondii in domestic cats, worldwide, has been estimated to be around 30–40%.[127] In the United States, no official national estimate has been made, but local surveys have shown levels varied between 16% and 80%.[127] A 2012 survey of 445 purebred pet cats and 45 shelter cats in Finland found an overall seroprevalence of 48.4%.[128] A 2010 survey of feral cats from Giza, Egypt, found an overall seroprevalence of 97.4%.[129] Another survey from Colombia showed the seroprevalence of 89.3% whereas a Chinese study showed seroprevalence of 2.1%.[130]
T. gondii infection rates in domestic cats vary widely depending on the cats' diets and lifestyles.[131] Feral cats that hunt for their food are more likely to be infected than domestic cats. The prevalence of T. gondii in cat populations depends on the availability of infected birds and small mammals,[132] but often this prey is abundant.
Most infected cats will shed oocysts only once in their lifetimes, for a period of about one to two weeks.[127] Although this period of shedding is quite transient, millions of oocysts can be shed, with each oocyst capable of spreading and surviving for months.[127] An estimated 1% of cats at any given time are actively shedding oocysts.[13]
It is difficult to control the cat population with the infected oocysts due to lack of an effective vaccine. This remains a challenge in most cases and the programs that are readily available are questionable in efficacy.[133]
Marine mammals
A University of California, Davis study of dead sea otters collected from 1998 to 2004 found toxoplasmosis was the cause of death for 13% of the animals.[134] Proximity to freshwater outflows into the ocean was a major risk factor. Ingestion of oocysts from cat feces is considered to be the most likely ultimate source.[135] Surface runoff containing wild cat feces and litter from domestic cats flushed down toilets are possible sources of oocysts.[136] The parasites have been found in dolphins and whales.[137] Researchers Black and Massie believe anchovies, which travel from estuaries into the open ocean, may be helping to spread the disease.[138]
Giant panda
Toxoplasma gondii has been reported as the cause of death of a Giant panda kept in a zoo in China, who died in 2014 of acute gastroenteritis and respiratory disease.[113] Although seemingly anecdotal, this report emphasizes that all warm-bloodied species are likely to be infected by T. gondii, including endangered species such as the Giant panda.
Research

Chronic infection with T. gondii has traditionally been considered asymptomatic in people with normal immune function.
Some evidence suggests latent infection may subtly influence a range of human behaviors and tendencies, and infection may alter the susceptibility to or intensity of a number of affective, psychiatric, or neurological disorders.[139] Research has linked toxoplasmosis with schizophrenia.[140]
Latent T. gondii infection in humans has been associated with a higher incidence of automobile accidents, potentially due to impaired psychomotor performance or enhanced risk-taking personality profiles.[139] Moreover, correlations have been found between positive antibody titers to T. gondii and OCD, Parkinson's disease, Alzheimer's disease, suicide in people with mood disorders, and bipolar disorder.[139] Positive antibody titers to T. gondii have been shown to be not correlative with major depression or dysthymia.[141] Although there is a correlation between T. gondii infection and many psychological disorders, scientists are still trying to find the cause on a cellular level.
A 2016 study using the Dunedin cohort found that "there was little evidence that T. gondii was related to increased risk of psychiatric disorder, poor impulse control, personality aberrations or neurocognitive impairment".[142]
Schizophrenia
Some evidence links T. gondii to schizophrenia.[58] Two 2012 meta-analysis found the rates of antibodies to T.gondii in schizophrenia was 2.7 times higher than that in control populations.[143][144] T.gondii antibody positivity was therefore considered an intermediate risk factor in relation to other known risk factors.[143]
Cautions that were noted include that the antibody tests do not detect toxoplasmosis directly, most people with schizophrenia do not have antibodies for toxoplasmosis, and publication bias might exist.[144]
The majority of these studies tested people already diagnosed with schizophrenia for T. gondii antibodies, associations between T. gondii and schizophrenia have been found prior to the onset of schizophrenia symptoms.[58] Studies attempting to explain the sex differences in schizophrenia onset have suggested it can be accounted for by a second peak of T.gondii infection incidence occurring from ages 25–30 observed in females only.[145] People with schizophrenia and T. gondii antibodies show a higher mortality rate than schizophrenics testing seronegative.[57] Although a mechanism supporting the association between schizophrenia and T.gondii infection is unclear, studies have attempted to discern a molecular mechanism to explain the correlation.[145]
Studies have demonstrated that antipsychotic drugs used in the treatment of schizophrenia appear to inhibit the replication of T. gondii tachyzoites in cell culture.[58]
Traffic accidents
In addition to a correlation with psychological disorders such as OCD, schizophrenia, and bipolar disorder, T. gondii can also lead to infected people having a higher risk of being in a car accident than uninfected individuals.[57] A study in the Czech Republic found that latent toxoplasmosis patients were involved in accidents 2.65 times more often than people without toxoplasmosis infection.[57] The risk also increased with significantly higher amounts of T. gondii antibodies in the host cells.[57]
Limitations of correlation analysis
In most of the current studies where positive correlations have been found between T. gondii antibody titers and certain behavioral traits or neurological disorders, T. gondii seropositivity tests are conducted after the onset of the examined disease or behavioral trait; that is, it is often unclear whether infection with the parasite increases the chances of having a certain trait or disorder, or if having a certain trait or disorder increases the chances of becoming infected with the parasite.[146] Groups of individuals with certain behavioral traits or neurological disorders may share certain behavioral tendencies that increase the likelihood of exposure to and infection with T. gondii; as a result, it is difficult to confirm causal relationships between T. gondii infections and associated neurological disorders or behavioral traits.[146] Provided there is in fact an etiological link between T. gondii and schizophrenia, studies have yet to determine why some individuals with latent toxoplasmosis develop schizophrenia while others do not, however, some plausible explanations include differing genetic susceptibility, parasite strain differences, and differences in the route of the acquired T.gondii infection.[147]
Multiple sclerosis
There is a negative association between an infection with the parasite T. gondii and multiple sclerosis, therefore, researchers have concluded that toxoplasmosis infection could be considered a protective factor.[148]
See also
References
- 1 2 3 "Parasites - Toxoplasmosis (Toxoplasma infection) Epidemiology & Risk Factors". March 26, 2015. Retrieved 22 August 2015.
- 1 2 3 4 5 6 7 Hunter, CA; Sibley, LD (November 2012). "Modulation of innate immunity by Toxoplasma gondii virulence effectors". Nature Reviews Microbiology. 10 (11): 766–78. doi:10.1038/nrmicro2858. PMID 23070557.
- 1 2 "Parasites - Toxoplasmosis (Toxoplasma infection) Disease". July 10, 2014. Retrieved 22 August 2015.
- ↑ "Parasites - Toxoplasmosis (Toxoplasma infection) Biology". March 17, 2015. Retrieved 22 August 2015.
- ↑ "Parasites - Toxoplasmosis (Toxoplasma infection) Diagnosis". January 10, 2013. Retrieved 22 August 2015.
- ↑ "Parasites - Toxoplasmosis (Toxoplasma infection) Prevention & Control". January 10, 2013. Retrieved 22 August 2015.
- ↑ "Parasites - Toxoplasmosis (Toxoplasma infection) Resources for Health Professionals". April 14, 2014. Retrieved 22 August 2015.
- 1 2 3 4 Flegr J, Prandota J, Sovičková M, Israili ZH (March 2014). "Toxoplasmosis--a global threat. Correlation of latent toxoplasmosis with specific disease burden in a set of 88 countries". PLoS ONE. 9 (3): e90203. doi:10.1371/journal.pone.0090203. PMC 3963851
 . PMID 24662942.
. PMID 24662942. Toxoplasmosis is becoming a global health hazard as it infects 30-50% of the world human population.
- 1 2 3 4 Jones JL, Parise ME, Fiore AE (2014). "Neglected parasitic infections in the United States: toxoplasmosis". Am. J. Trop. Med. Hyg. 90 (5): 794–9. doi:10.4269/ajtmh.13-0722. PMC 4015566
 . PMID 24808246.
. PMID 24808246. - ↑ Torgerson, PR; Mastroiacovo, P (1 July 2013). "The global burden of congenital toxoplasmosis: a systematic review.". Bulletin of the World Health Organization. 91 (7): 501–8. doi:10.2471/blt.12.111732. PMID 23825877.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 Ferguson DJ (2009). "Toxoplasma gondii: 1908-2008, homage to Nicolle, Manceaux and Splendore". Memórias Do Instituto Oswaldo Cruz. 104 (2): 133–48. doi:10.1590/S0074-02762009000200003. PMID 19430635.
- 1 2 Dupont CD, Christian DA, Hunter CA (2012). "Immune response and immunopathology during toxoplasmosis". Seminars in Immunopathology. 34 (6): 793–813. doi:10.1007/s00281-012-0339-3. PMC 3498595
 . PMID 22955326.
. PMID 22955326. - 1 2 3 4 5 6 7 8 9 Dubey JP, Jones JL (September 2008). "Toxoplasma gondii infection in humans and animals in the United States". International Journal for Parasitology. 38 (11): 1257–78. doi:10.1016/j.ijpara.2008.03.007. PMID 18508057.
- 1 2 "toxoplasmosis".
- ↑ Jones JL, Kruszon-Moran D, Wilson M, McQuillan G, Navin T, McAuley JB (2001). "Toxoplasma gondii infection in the United States: seroprevalence and risk factors". American Journal of Epidemiology. 154 (4): 357–65. doi:10.1093/aje/154.4.357. PMID 11495859.
- ↑ Paul M (1 July 1999). "Immunoglobulin G Avidity in Diagnosis of Toxoplasmic Lymphadenopathy and Ocular Toxoplasmosis". Clin. Diagn. Lab. Immunol. 6 (4): 514–8. PMC 95718
 . PMID 10391853.
. PMID 10391853. - ↑ "Lymphadenopathy" (PDF). UK Neqas Micro. Retrieved 2016-04-12.
- ↑ "CDC Parasites – Toxoplasmosis (Toxoplasma infection) – Disease". Retrieved 12 March 2013.
- ↑ Dubey JP, Hodgin EC, Hamir AN (2006). "Acute fatal toxoplasmosis in squirrels (Sciurus carolensis) with bradyzoites in visceral tissues". The Journal of Parasitology. 92 (3): 658–9. doi:10.1645/GE-749R.1. PMID 16884019.
- ↑ Nawaz Khan, A (2015). "Imaging in CNS Toxoplasmosis". Medscape web site.
- 1 2 Blanchard N, Dunay IR, Schlüter D (2015). "Persistence of Toxoplasma gondii in the central nervous system: a fine-tuned balance between the parasite, the brain and the immune system". Parasite Immunol. 37 (3): 150–158. doi:10.1111/pim.12173. PMID 25573476.
The seroprevalence of T. gondii in humans varies between 10 and 70% worldwide, depending on the region and increases significantly with age. Upon infection, the parasites persist as intraneuronal cysts in the central nervous system (CNS) for the lifetime of the host (1, Figure 1). Until recently, parasite persistence in healthy individuals was regarded as clinically asymptomatic. However, in the last decade, several reports have indicated that chronic cerebral toxoplasmosis may impact on the behaviour of its host (2).
- ↑ Randall Parker: Humans Get Personality Altering Infections From Cats. September 30, 2003
- 1 2 Parlog A, Schlüter D, Dunay IR (March 2015). "Toxoplasma gondii-induced neuronal alterations". Parasite Immunol. 37 (3): 159–170. doi:10.1111/pim.12157. PMID 25376390.
The zoonotic pathogen Toxoplasma gondii infects over 30% of the human population. The intracellular parasite can persist lifelong in the CNS within neurons modifying their function and structure, thus leading to specific behavioural changes of the host. ... Furthermore, investigations of the human population have correlated Toxoplasma seropositivity with changes in neurological functions; however, the complex underlying mechanisms of the subtle behavioural alteration are still not fully understood. The parasites are able to induce direct modifications in the infected cells, for example by altering dopamine metabolism, by functionally silencing neurons as well as by hindering apoptosis.
- ↑ Pappas, G; Roussos, N; Falagas, ME (October 2009). "Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis.". International Journal for Parasitology. 39 (12): 1385–94. doi:10.1016/j.ijpara.2009.04.003. PMID 19433092.
- ↑ Cook TB, Brenner LA, Cloninger CR, et al. (2015). ""Latent" infection with Toxoplasma gondii: association with trait aggression and impulsivity in healthy adults". J Psychiatr Res. 60: 87–94. doi:10.1016/j.jpsychires.2014.09.019. PMID 25306262.
- ↑ Hurley RA, Taber KH (2012). "Latent Toxoplasmosis gondii: emerging evidence for influences on neuropsychiatric disorders". J Neuropsychiatry Clin Neurosci. 24 (4): 376–83. doi:10.1176/appi.neuropsych.12100234. PMID 23224444.
Nine of eleven studies using the Cattell’s 16-Personality Factor self-report questionnaire found significant and consistent results for both genders. Seropositive men overall had lower regard for rules and higher vigilance (suspicious, jealous, rigid/inflexible) than seronegative men. In contrast, seropositive women had greater regard for rules and higher warmth than seronegative women. Both seropositive genders were more anxious than matched healthy-comparison subjects. ... Behavioral observations and interviews were completed to ascertain whether the gender differences found in self-report measures were replicated by objective measures. Seropositive men scored significantly lower than seronegative men on Self-Control, Clothes Tidiness, and Relationships. The differences were less impressive for the seropositive women, with only trends toward higher scores on Self-Control and Clothes Tidiness as compared with seronegative women. The authors view the study results as objective confirmation that T. gondii presence can change a human host’s behaviors.
- ↑ Barakat AM, Salem LM, El-Newishy AM, Shaapan RM, El-Mahllawy EK (2012). "Zoonotic chicken toxoplasmosis in some Egyptians governorates". Pakistan Journal of Biological Sciences: PJBS. 15 (17): 821–6. doi:10.3923/pjbs.2012.821.826. PMID 24163965.
- ↑ Klaus, Sidney N.; Shoshana Frankenburg, and A. Damian Dhar (2003). "Chapter 235: Leishmaniasis and Other Protozoan Infections". In Freedberg; et al. Fitzpatrick's Dermatology in General Medicine. (6th ed.). McGraw-Hill. ISBN 0-07-138067-1.
- 1 2 3 Robert-Gangneux, F.; Darde, M.-L. (2012). "Epidemiology of and Diagnostic Strategies for Toxoplasmosis". Clinical Microbiology Reviews. 25 (2): 264–296. doi:10.1128/CMR.05013-11. ISSN 0893-8512. PMID 22491772.
- 1 2 3 4 5 Miller CM; Boulter NR; Ikin RJ; Smith NC (January 2009). "The immunobiology of the innate response to Toxoplasma gondii". International Journal for Parasitology. 39 (1): 23–39. doi:10.1016/j.ijpara.2008.08.002. PMID 18775432.
- 1 2 3 Martens S; Parvanova I; Zerrahn J; Griffiths G; Schell G; Reichmann G; Howard JC (November 2005). "Disruption of Toxoplasma gondii parasitophorous vacuoles by the mouse p47-resistance GTPases". PLoS Pathogens. 1 (3): e24. doi:10.1371/journal.ppat.0010024. PMC 1287907
 . PMID 16304607.
. PMID 16304607. - 1 2 Denkers, EY; Schneider, AG; Cohen, AB; Butcher, BA (2012). "Phagocyte responses to protozoan infection and how Toxoplasma gondii meets the challenge". PLoS Pathogens. 8 (8): e1002794. doi:10.1371/journal.ppat.1002794. PMC 3410898
 . PMID 22876173.
. PMID 22876173. - 1 2 3 Hippe D, Weber A, Zhou L, Chang DC, Häcker G, Lüder CG (2009). "Toxoplasma gondii infection confers resistance against BimS-induced apoptosis by preventing the activation and mitochondrial targeting of pro-apoptotic Bax". Journal of Cell Science. 122 (Pt 19): 3511–21. doi:10.1242/jcs.050963. PMID 19737817.
- 1 2 3 Wang Y, Weiss LM, Orlofsky A (2009). "Host cell autophagy is induced by Toxoplasma gondii and contributes to parasite growth". The Journal of Biological Chemistry. 284 (3): 1694–701. doi:10.1074/jbc.M807890200. PMC 2615531
 . PMID 19028680.
. PMID 19028680. - 1 2 Laliberté J, Carruthers VB (2008). "Host cell manipulation by the human pathogen Toxoplasma gondii". Cellular and Molecular Life Sciences : CMLS. 65 (12): 1900–15. doi:10.1007/s00018-008-7556-x. PMC 2662853
 . PMID 18327664.
. PMID 18327664. - ↑ Weiss, Louis M.; Dubey, Jitender. P. (2009). "Toxoplasmosis: A history of clinical observations". International Journal for Parasitology. 39 (8): 895–901. doi:10.1016/j.ijpara.2009.02.004. ISSN 0020-7519. PMC 2704023
 . PMID 19217908.
. PMID 19217908. - ↑ Toxoplasma gondii: the model apicomplexan: perspectives and methods. Elsevier/Academic Press. 2007. ISBN 9780123695420.
- ↑ "Toxoplasmosis". Centers of Disease Control and Prevention. 2004-11-22.
- ↑ Jones JL, Dubey JP (2012). "Foodborne toxoplasmosis". Clinical Infectious Diseases. 55 (6): 845–51. doi:10.1093/cid/cis508. PMID 22618566.
- ↑ North Carolina Department of Agriculture & Consumer Services
- ↑ "Parasites – Toxoplasmosis (Toxoplasma infection)". Centers of Disease Control and Prevention. 2011-04-05.
- ↑ Assadi-Rad AM, New JC, Patton S (5 April 1995). "Risk factors associated with transmission of Toxoplasma gondii to sows kept in different management systems in Tennessee.". Vet. Parasitol. 57: 289–97. doi:10.1016/0304-4017(94)00677-5. PMID 7660566.
- 1 2 Sterkers Y, Ribot J, Albaba S, Issert E, Bastien P, Pratlong F (2011). "Diagnosis of congenital toxoplasmosis by polymerase chain reaction on neonatal peripheral blood". Diagnostic Microbiology and Infectious Disease. 71 (2): 174–6. doi:10.1016/j.diagmicrobio.2011.06.006. PMID 21856107.
- 1 2 Torgerson, Paul R; Mastroiacovo, Pierpaolo (2013). "The global burden of congenital toxoplasmosis: a systematic review". Bulletin of the World Health Organization. 91 (7): 501–508. doi:10.2471/BLT.12.111732. ISSN 0042-9686. PMID 23825877.
- 1 2 3 Di Mario, S; Basevi, V; Gagliotti, C; Spettoli, D; Gori, G; D'Amico, R; Magrini, N (23 October 2015). "Prenatal education for congenital toxoplasmosis.". The Cochrane database of systematic reviews. 10: CD006171. doi:10.1002/14651858.CD006171.pub4. PMID 26493047.
- ↑ "Circular Normativa sobre Cuidados Pré-Concepcionais – Direcção-Geral de Saúde" (PDF). Archived from the original (PDF) on 2011-07-16.
- 1 2 3 4 Sukthana Y (March 2006). "Toxoplasmosis: beyond animals to humans". Trends Parasitol. 22 (3): 137–42. doi:10.1016/j.pt.2006.01.007. PMID 16446116.
- ↑ Archived August 24, 2011, at the Wayback Machine.
- 1 2 De Paschale M, Agrappi C, Clerici P, Mirri P, Manco MT, Cavallari S, Viganò EF (2008). "Seroprevalence and incidence of Toxoplasma gondii infection in the Legnano area of Italy". Clinical Microbiology and Infection. 14 (2): 186–9. doi:10.1111/j.1469-0691.2007.01883.x. PMID 18034857.
- 1 2 3 4 Kapperud G, Jenum PA, Stray-Pedersen B, Melby KK, Eskild A, Eng J (1996). "Risk factors for Toxoplasma gondii infection in pregnancy. Results of a prospective case-control study in Norway". American Journal of Epidemiology. 144 (4): 405–12. doi:10.1093/oxfordjournals.aje.a008942. PMID 8712198.
- 1 2 3 4 Hill D, Dubey JP (2002). "Toxoplasma gondii: transmission, diagnosis and prevention". Clinical Microbiology and Infection. 8 (10): 634–40. doi:10.1046/j.1469-0691.2002.00485.x. PMID 12390281.
- ↑ Cook AJ, Gilbert RE, Buffolano W, Zufferey J, Petersen E, Jenum PA, Foulon W, Semprini AE, Dunn DT (Jul 15, 2000). "Sources of toxoplasma infection in pregnant women: European multicentre case-control study. European Research Network on Congenital Toxoplasmosis". BMJ (Clinical research ed.). 321 (7254): 142–7. doi:10.1136/bmj.321.7254.142. PMC 27431
 . PMID 10894691.
. PMID 10894691. - ↑ Bobić B, Jevremović I, Marinković J, Sibalić D, Djurković-Djaković O (September 1998). "Risk factors for Toxoplasma infection in a reproductive age female population in the area of Belgrade, Yugoslavia". European journal of epidemiology. 14 (6): 605–10. doi:10.1023/A:1007461225944. PMID 9794128.
- ↑ Jones JL, Dargelas V, Roberts J, Press C, Remington JS, Montoya JG (2009). "Risk Factors forToxoplasma gondiiInfection in the United States". Clinical Infectious Diseases. 49 (6): 878–884. doi:10.1086/605433. PMID 19663709.
- ↑ Kanková S, Sulc J, Nouzová K, Fajfrlík K, Frynta D, Flegr J (2007). "Women infected with parasite Toxoplasma have more sons". Die Naturwissenschaften. 94 (2): 122–7. doi:10.1007/s00114-006-0166-2. PMID 17028886.
- ↑ Ian Sample, science correspondent (2006-10-12). "Pregnant women infected by cat parasite more likely to give birth to boys, say researchers | Science". London: The Guardian. Retrieved 2013-02-14.
- 1 2 3 4 5 6 7 Dalimi A, Abdoli A (2011). "Latent Toxoplasmosis and Human". Iranian Journal of Parasitology. 7 (1): 1–17.
- 1 2 3 4 5 Webster JP, McConkey GA (June 2010). "Toxoplasma gondii-altered host behaviour: clues as to mechanism of action". Folia parasitologica. 57 (2): 95–104. doi:10.14411/fp.2010.012. PMID 20608471.
- ↑ Webster JP (May 2007). "The effect of Toxoplasma gondii on animal behavior: playing cat and mouse" (PDF). Schizophrenia bulletin. 33 (3): 752–6. doi:10.1093/schbul/sbl073. PMC 2526137
 . PMID 17218613.
. PMID 17218613. - 1 2 Berdoy M, Webster JP, Macdonald DW (Aug 7, 2000). "Fatal attraction in rats infected with Toxoplasma gondii". Proceedings. Biological sciences / the Royal Society. 267 (1452): 1591–4. doi:10.1098/rspb.2000.1182. PMC 1690701
 . PMID 11007336.
. PMID 11007336. - ↑ Vyas A, Kim SK, Giacomini N, Boothroyd JC, Sapolsky RM (Apr 10, 2007). "Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors". Proceedings of the National Academy of Sciences of the United States of America. 104 (15): 6442–7. Bibcode:2007PNAS..104.6442V. doi:10.1073/pnas.0608310104. PMC 1851063
 . PMID 17404235.
. PMID 17404235. - ↑ Xiao J, Kannan G, Jones-Brando L, Brannock C, Krasnova IN, Cadet JL, Pletnikov M, Yolken RH (Mar 29, 2012). "Sex-specific changes in gene expression and behavior induced by chronic Toxoplasma infection in mice". Neuroscience. 206: 39–48. doi:10.1016/j.neuroscience.2011.12.051. PMID 22240252.
- ↑ Lamberton PH, Donnelly CA, Webster JP (September 2008). "Specificity of theToxoplasma gondii-altered behaviour to definitive versus non-definitive host predation risk". Parasitology. 135 (10): 1143–50. doi:10.1017/S0031182008004666. PMID 18620624.
- 1 2 Hari Dass SA, Vyas A (December 2014). "Toxoplasma gondii infection reduces predator aversion in rats through epigenetic modulation in the host medial amygdala". Mol. Ecol. 23 (24): 6114–6122. doi:10.1111/mec.12888. PMID 25142402.
- 1 2 Flegr J, Markoš A (December 2014). "Masterpiece of epigenetic engineering - how Toxoplasma gondii reprogrammes host brains to change fear to sexual attraction". Mol. Ecol. 23 (24): 5934–5936. doi:10.1111/mec.13006. PMID 25532868.
- ↑ McCowan TJ, Dhasarathy A, Carvelli L (February 2015). "The Epigenetic Mechanisms of Amphetamine" (PDF). J. Addict. Prev. Avens Publishing Group (S1): 1–7. ISSN 2330-2178. Retrieved 30 April 2015.
Epigenetic modifications caused by addictive drugs play an important role in neuronal plasticity and in drug-induced behavioral responses. Although few studies have investigated the effects of AMPH on gene regulation (Table 1), current data suggest that AMPH acts at multiple levels to alter histone/DNA interaction and to recruit transcription factors which ultimately cause repression of some genes and activation of other genes. Importantly, some studies have also correlated the epigenetic regulation induced by AMPH with the behavioral outcomes caused by this drug, suggesting therefore that epigenetics remodeling underlies the behavioral changes induced by AMPH. If this proves to be true, the use of specific drugs that inhibit histone acetylation, methylation or DNA methylation might be an important therapeutic alternative to prevent and/or reverse AMPH addiction and mitigate the side effects generate by AMPH when used to treat ADHD.
- ↑ Walker DM, Cates HM, Heller EA, Nestler EJ (February 2015). "Regulation of chromatin states by drugs of abuse". Curr. Opin. Neurobiol. 30: 112–121. doi:10.1016/j.conb.2014.11.002. PMID 25486626.
- ↑ Nestler EJ (January 2014). "Epigenetic mechanisms of drug addiction". Neuropharmacology. 76 Pt B: 259–268. doi:10.1016/j.neuropharm.2013.04.004. PMC 3766384
 . PMID 23643695.
. PMID 23643695. Short-term increases in histone acetylation generally promote behavioral responses to the drugs, while sustained increases oppose cocaine’s effects, based on the actions of systemic or intra-NAc administration of HDAC inhibitors. ... Genetic or pharmacological blockade of G9a in the NAc potentiates behavioral responses to cocaine and opiates, whereas increasing G9a function exerts the opposite effect (Maze et al., 2010; Sun et al., 2012a). Such drug-induced downregulation of G9a and H3K9me2 also sensitizes animals to the deleterious effects of subsequent chronic stress (Covington et al., 2011). Downregulation of G9a increases the dendritic arborization of NAc neurons, and is associated with increased expression of numerous proteins implicated in synaptic function, which directly connects altered G9a/H3K9me2 in the synaptic plasticity associated with addiction (Maze et al., 2010).
G9a appears to be a critical control point for epigenetic regulation in NAc, as we know it functions in two negative feedback loops. It opposes the induction of ΔFosB, a long-lasting transcription factor important for drug addiction (Robison and Nestler, 2011), while ΔFosB in turn suppresses G9a expression (Maze et al., 2010; Sun et al., 2012a). ... Also, G9a is induced in NAc upon prolonged HDAC inhibition, which explains the paradoxical attenuation of cocaine’s behavioral effects seen under these conditions, as noted above (Kennedy et al., 2013). GABAA receptor subunit genes are among those that are controlled by this feedback loop. Thus, chronic cocaine, or prolonged HDAC inhibition, induces several GABAA receptor subunits in NAc, which is associated with increased frequency of inhibitory postsynaptic currents (IPSCs). In striking contrast, combined exposure to cocaine and HDAC inhibition, which triggers the induction of G9a and increased global levels of H3K9me2, leads to blockade of GABAA receptor and IPSC regulation. - ↑ Vanagas L, Jeffers V, Bogado SS, Dalmasso MC, Sullivan WJ, Angel SO (October 2012). "Toxoplasma histone acetylation remodelers as novel drug targets". Expert Rev Anti Infect Ther. 10 (10): 1189–1201. doi:10.1586/eri.12.100. PMC 3581047
 . PMID 23199404.
. PMID 23199404. - ↑ Bouchut A, Chawla AR, Jeffers V, Hudmon A, Sullivan WJ (2015). "Proteome-wide lysine acetylation in cortical astrocytes and alterations that occur during infection with brain parasite Toxoplasma gondii". PLoS ONE. 10 (3): e0117966. doi:10.1371/journal.pone.0117966. PMC 4364782
 . PMID 25786129.
. PMID 25786129. - ↑ McConkey GA, Martin HL, Bristow GC, Webster JP (Jan 1, 2013). "Toxoplasma gondii infection and behaviour – location, location, location?". The Journal of Experimental Biology. 216 (Pt 1): 113–9. doi:10.1242/jeb.074153. PMC 3515035
 . PMID 23225873.
. PMID 23225873. - 1 2 Afonso C, Paixão VB, Costa RM (2012). Hakimi, ed. "Chronic Toxoplasma infection modifies the structure and the risk of host behavior". PLoS ONE. 7 (3): e32489. Bibcode:2012PLoSO...732489A. doi:10.1371/journal.pone.0032489. PMC 3303785
 . PMID 22431975.
. PMID 22431975. - ↑ Gonzalez LE, Rojnik B, Urrea F, Urdaneta H, Petrosino P, Colasante C, Pino S, Hernandez L (Feb 12, 2007). "Toxoplasma gondii infection lower anxiety as measured in the plus-maze and social interaction tests in rats A behavioral analysis". Behavioural Brain Research. 177 (1): 70–9. doi:10.1016/j.bbr.2006.11.012. PMID 17169442.
- ↑ Switaj K, Master A, Skrzypczak M, Zaborowski P (2005). "Recent trends in molecular diagnostics for Toxoplasma gondii infections". Clin. Microbiol. Infect. 11 (3): 170–6. doi:10.1111/j.1469-0691.2004.01073.x. PMID 15715713.
- 1 2 3 4 Montoya JG (2002). "Laboratory diagnosis of Toxoplasma gondii infection and toxoplasmosis". The Journal of Infectious Diseases. 185 Suppl 1: S73–82. doi:10.1086/338827. PMID 11865443.
- ↑ Remington, J. S.; Thulliez, P.; Montoya, J. G. (2004). "Recent Developments for Diagnosis of Toxoplasmosis". Journal of Clinical Microbiology. 42 (3): 941–945. doi:10.1128/JCM.42.3.941-945.2004. ISSN 0095-1137.
- ↑ Sensini, A. (2006). "Toxoplasma gondii infection in pregnancy: opportunities and pitfalls of serological diagnosis". Clinical Microbiology and Infection. 12 (6): 504–512. doi:10.1111/j.1469-0691.2006.01444.x. ISSN 1198-743X.
- 1 2 3 4 5 6 Lin MH, Chen TC, Kuo TT, Tseng CC, Tseng CP (2000). "Real-time PCR for quantitative detection of Toxoplasma gondii". Journal of Clinical Microbiology. 38 (11): 4121–5. PMC 87551
 . PMID 11060078.
. PMID 11060078. - ↑ Doggett JS, Nilsen A, Forquer I, Wegmann KW, Jones-Brando L, Yolken RH, Bordón C, Charman SA, Katneni K, Schultz T, Burrows JN, Hinrichs DJ, Meunier B, Carruthers VB, Riscoe MK (2012). "Endochin-like quinolones are highly efficacious against acute and latent experimental toxoplasmosis". Proceedings of the National Academy of Sciences of the United States of America. 109 (39): 15936–41. doi:10.1073/pnas.1208069109. PMC 3465437
 . PMID 23019377.
. PMID 23019377. - ↑ "CDC - Toxoplasmosis - Resources for Health Professionals". www.cdc.gov. Retrieved 5 December 2016.
- ↑ "Toxoplasmosis – treatment key research". NAM & aidsmap. 2005-11-02.
- ↑ Djurković-Djaković O, Milenković V, Nikolić A, Bobić B, Grujić J (2002). "Efficacy of atovaquone combined with clindamycin against murine infection with a cystogenic (Me49) strain of Toxoplasma gondii" (PDF). J Antimicrob Chemother. 50 (6): 981–7. doi:10.1093/jac/dkf251. PMID 12461021.
- ↑ Jones J, Lopez A, Wilson M (2003). "Congenital toxoplasmosis". Am Fam Physician. 67 (10): 2131–8. PMID 12776962.
- ↑ McLeod R, Kieffer F, Sautter M, Hosten T, Pelloux H (2009). "Why prevent, diagnose and treat congenital toxoplasmosis?". Mem. Inst. Oswaldo Cruz. 104 (2): 320–44. doi:10.1590/s0074-02762009000200029. PMC 2735102
 . PMID 19430661.
. PMID 19430661. - 1 2 3 Pappas G, Roussos N, Falagas ME (October 2009). "Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis". International Journal for Parasitology. 39 (12): 1385–94. doi:10.1016/j.ijpara.2009.04.003. PMID 19433092.
- 1 2 Jones JL, Kruszon-Moran D, Sanders-Lewis K, Wilson M (September 2007). "Toxoplasma gondii infection in the United States, 1999 2004, decline from the prior decade". The American journal of tropical medicine and hygiene. 77 (3): 405–10. PMID 17827351.
- ↑ Sibley LD; Khan A; Ajioka JW; Rosenthal BM (2009). "Genetic diversity of Toxoplasma gondii in animals and humans". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 364 (1530): 2749–2761. doi:10.1098/rstb.2009.0087. PMID 19687043.
- ↑ "CDC: Parasites – Toxoplasmosis (Toxoplasma infection) – Pregnant Women". Retrieved 13 March 2013.
- ↑ Dubey JP, Frenkel JK (May 1998). "Toxoplasmosis of rats: a review, with considerations of their value as an animal model and their possible role in epidemiology". Veterinary parasitology. 77 (1): 1–32. doi:10.1016/S0304-4017(97)00227-6. PMID 9652380.
- 1 2 3 4 5 6 7 8 Weiss LM, Dubey JP (2009). "Toxoplasmosis: A history of clinical observations". International Journal for Parasitology. 39 (8): 895–901. doi:10.1016/j.ijpara.2009.02.004. PMC 2704023
 . PMID 19217908.
. PMID 19217908. - ↑ "Laboratory Tests For The Diagnosis Of Toxoplasmosis". Toxoplasma Serology Laboratory.
- ↑ "How Your Cat Is Making You Crazy - Kathleen McAuliffe". The Atlantic. 2012-02-06. Retrieved 2013-06-03.
- ↑ "'Cat Lady' Conundrum - Rebecca Skloot". The New York Times. 2007-12-09.
- ↑ "Your cat is making you crazy, says scientist - Lorianna De Giorgio". Toronto Star. 2012-02-18.
- ↑ "Why Your Cat May Be Making You Crazy". Animal Planet. 2012-03-01.
- ↑ Fox, Stuart (2010-03-09). "Gold Nanoparticles and Lasers Kill the Brain Parasite That Causes "Crazy Cat Lady" Syndrome". Popsci.
- ↑ Kapperud, Georg; Jenum, Pal A.; Stray-Pedersen, Babill; Melby, Kjetil K.; Eskild, Anne; Eng, Jan (1996-08-15). "Risk Factors for Toxoplasma gondii Infection in Pregnancy Results of a Prospective Case-Control Study in Norway". American Journal of Epidemiology. 144 (4): 405–412. doi:10.1093/oxfordjournals.aje.a008942. ISSN 0002-9262. PMID 8712198.
- ↑ Cook, A. J. C.; Holliman, Richard; Gilbert, R. E.; Buffolano, W.; Zufferey, J.; Petersen, E.; Jenum, P. A.; Foulon, W.; Semprini, A. E. (2000-07-15). "Sources of toxoplasma infection in pregnant women: European multicentre case-control studyCommentary: Congenital toxoplasmosis—further thought for food". BMJ. 321 (7254): 142–147. doi:10.1136/bmj.321.7254.142. ISSN 0959-8138. PMC 27431
 . PMID 10894691.
. PMID 10894691. - ↑ Torrey, E.; Simmons, Wendy; Yolken, Robert (June 2015). "Is childhood cat ownership a risk factor for schizophrenia later in life?". Schizophrenia Research. 165 (1): 1–2. doi:10.1016/j.schres.2015.03.036.
- ↑ Kathleen McAuliffe (March 2012). "How Your Cat is Making You Crazy". The Atlantic.
- ↑ Flegr Jaroslav. "Effects of Toxoplasma on Human Behavior". Schizophrenia Bulletin. 33 (3): 757–760. doi:10.1093/schbul/sbl074. PMC 2526142
 . PMID 17218612.
. PMID 17218612. - ↑ Arthur Ashe, Tennis Star, is Dead at 49 Archived December 10, 2008, at the Wayback Machine. New York Times (02/08/93)
- ↑ Merritt Butrick, A Biography Angelfire.com, accessdate Mar 18, 2011
- ↑ "Pedro Zamora Biography :: HIV Aids Activism Biography".
- ↑ "The Face That Defined AIDS".
- ↑ "Pregnancy superfoods revealed". BBC News. January 10, 2001. Retrieved May 25, 2010.
- ↑ "Olympics bid Coes finest race". The Times. London. June 26, 2005. Retrieved May 25, 2010.
- ↑ Brody, Jane E. (27 October 1982). "PERSONAL HEALTH". New York Times.
- ↑ "Topic 33. Coccidia and Cryptosporidium spp". Biology 625: Animal Parasitology. Kent State Parasitology Lab. October 24, 2005. Retrieved 2006-10-01.
- ↑ "The infinite appeal of the Wealdstone Raider [@BabboPieta Feature". 101 Great Goals. 2014-12-16. Retrieved 2015-10-18.
- ↑ Welsh, Irvine (2008-12-05). Trainspotting. Random House. p. 358. ISBN 9781407019994.
- ↑ Rigoulet, Jacques; Hennache, Alain; Lagourette, Pierre; George, Catherine; Longeart, Loïc; Le Net, Jean-Loïc; Dubey, Jitender P. (2014). "Toxoplasmosis in a bar-shouldered dove (Geopelia humeralis) from the Zoo of Clères, France". Parasite. 21: 62. doi:10.1051/parasite/2014062. ISSN 1776-1042. PMC 4236686
 . PMID 25407506.
. PMID 25407506. 
- 1 2 Ma, Hongyu; Wang, Zedong; Wang, Chengdong; Li, Caiwu; Wei, Feng; Liu, Quan (2015). "Fatal Toxoplasma gondii infection in the giant panda". Parasite. 22: 30. doi:10.1051/parasite/2015030. ISSN 1776-1042. PMID 26514595.

- ↑ J.P Dubey (2010)
- ↑ Ekanayake DK, Rajapakse RP, Dubey JP, Dittus WP (2015-04-20). "Seroprevalence of Toxoplasma gondii in wild toque macaques (Macaca sinica) at Polonnaruwa, Sri Lanka". J. Parasitol. 90: 870–1. doi:10.1645/GE-291R. PMID 15357087.
- ↑ Chessa G, Chisu V, Porcu R, Masala G (2014). "Molecular characterization of Toxoplasma gondii Type II in sheep abortion in Sardinia, Italy". Parasite. 21: 6. doi:10.1051/parasite/2014007. PMC 3927306
 . PMID 24534616.
. PMID 24534616. 
- 1 2 3 4 Tenter AM, Heckeroth AR, Weiss LM (November 2000). "Toxoplasma gondii: from animals to humans". International Journal for Parasitology. 30 (12–13): 1217–58. doi:10.1016/S0020-7519(00)00124-7. PMC 3109627
 . PMID 11113252.
. PMID 11113252. - 1 2 3 4 Jones JL, Dubey JP (September 2012). "Foodborne toxoplasmosis". Clinical Infectious Diseases. 55 (6): 845–51. doi:10.1093/cid/cis508. PMID 22618566.
- ↑ J.P Dubey (2010) p. 145-151
- ↑ Dubey JP, Sundar N, Hill D, Velmurugan GV, Bandini LA, Kwok OC, Majumdar D, Su C (July 2008). "High prevalence and abundant atypical genotypes of Toxoplasma gondii isolated from lambs destined for human consumption in the USA". International Journal for Parasitology. 38 (8–9): 999–1006. doi:10.1016/j.ijpara.2007.11.012. PMID 18191859.
- ↑ Dubey JP, Rajendran C, Ferreira LR, Martins J, Kwok OC, Hill DE, Villena I, Zhou H, Su C, Jones JL (July 2011). "High prevalence and genotypes ofToxoplasma gondii isolated from goats, from a retail meat store, destined for human consumption in the USA". International Journal for Parasitology. 41 (8): 827–33. doi:10.1016/j.ijpara.2011.03.006. PMID 21515278.
- ↑ Dubey JP (February 2010). "Toxoplasma gondii infections in chickens (Gallus domesticus): prevalence, clinical disease, diagnosis and public health significance". Zoonoses and public health. 57 (1): 60–73. doi:10.1111/j.1863-2378.2009.01274.x. PMID 19744305.
- ↑ Louis M Weiss, Kami Kim (2011) p. 723
- ↑ Aroussi, Abdelkrim; Vignoles, Philippe; Dalmay, François; Wimel, Laurence; Dardé, Marie-Laure; Mercier, Aurélien; Ajzenberg, Daniel (2015). "Detection of Toxoplasma gondii DNA in horse meat from supermarkets in France and performance evaluation of two serological tests". Parasite. 22: 14. doi:10.1051/parasite/2015014. ISSN 1776-1042. PMC 4374124
 . PMID 25809058.
. PMID 25809058. 
- ↑ Dubey, Jitender P. (2008-12-01). "The history of Toxoplasma gondii--the first 100 years". The Journal of Eukaryotic Microbiology. 55 (6): 467–475. doi:10.1111/j.1550-7408.2008.00345.x. ISSN 1550-7408. PMID 19120791.
- ↑ Hutchison, WM (1965-04-29). "Experimental transmission of Toxoplasma gondii".
- 1 2 3 4 Elmore SA, Jones JL, Conrad PA, Patton S, Lindsay DS, Dubey JP (April 2010). "Toxoplasma gondii: epidemiology, feline clinical aspects, and prevention". Trends in parasitology. 26 (4): 190–6. doi:10.1016/j.pt.2010.01.009. PMID 20202907.
- ↑ Jokelainen P, Simola O, Rantanen E, Näreaho A, Lohi H, Sukura A (November 2012). "Feline toxoplasmosis in Finland: cross-sectional epidemiological study and case series study". Journal of veterinary diagnostic investigation : official publication of the American Association of Veterinary Laboratory Diagnosticians, Inc. 24 (6): 1115–24. doi:10.1177/1040638712461787. PMID 23012380.
- ↑ Al-Kappany YM, Rajendran C, Ferreira LR, Kwok OC, Abu-Elwafa SA, Hilali M, Dubey JP (December 2010). "High prevalence of toxoplasmosis in cats from Egypt: isolation of viable Toxoplasma gondii, tissue distribution, and isolate designation". The Journal of Parasitology. 96 (6): 1115–8. doi:10.1645/GE-2554.1. PMID 21158619.
- ↑ Dubey, JP (2010). Toxoplasmosis of Animals and Humans. ISBN 978-1-4200-9236-3.
- ↑ J.P Dubey (2010) p. 95
- ↑ J.P Dubey (2010) p. 46
- ↑ Andersen, Mark C.; Martin, Brent J.; Roemer, Gary W. (2004-12-15). "Use of matrix population models to estimate the efficacy of euthanasia versus trap-neuter-return for management of free-roaming cats". Journal of the American Veterinary Medical Association. 225 (12): 1871–1876. doi:10.2460/javma.2004.225.1871. ISSN 0003-1488. PMID 15643836.
- ↑ Conrad PA, Miller MA, Kreuder C, James ER, Mazet J, Dabritz H, Jessup DA, Gulland F, Grigg ME (2005). "Transmission of Toxoplasma: clues from the study of sea otters as sentinels of Toxoplasma gondii flow into the marine environment". Int J Parasitol. 35 (11–12): 1155–68. doi:10.1016/j.ijpara.2005.07.002. PMID 16157341.
- ↑ 17:30–22:00 "Treating Disease in the Developing World" Check
|url=value (help). Talk of the Nation Science Friday. National Public Radio. December 16, 2005. - ↑ "Parasite in cats killing sea otters". NOAA magazine. National Oceanic and Atmospheric Administration. 21 January 2003. Retrieved 24 November 2007.
- ↑ "3 Schizophrenia".
- ↑ Massie, Gloeta N.; Ware, Michael W.; Villegas, Eric N.; Black, Michael W. (2010). "Uptake and transmission of Toxoplasma gondii oocysts by migratory, filter-feeding fish". Veterinary Parasitology. 169 (3-4): 296–303. doi:10.1016/j.vetpar.2010.01.002. ISSN 0304-4017.
- 1 2 3 Webster JP, Kaushik M, Bristow GC, McConkey GA (Jan 1, 2013). "Toxoplasma gondii infection, from predation to schizophrenia: can animal behaviour help us understand human behaviour?". The Journal of Experimental Biology. 216 (Pt 1): 99–112. doi:10.1242/jeb.074716. PMC 3515034
 . PMID 23225872.
. PMID 23225872. - ↑ Fabiani, S; Pinto, B; Bonuccelli, U; Bruschi, F (15 April 2015). "Neurobiological studies on the relationship between toxoplasmosis and neuropsychiatric diseases.". Journal of the neurological sciences. 351 (1-2): 3–8. doi:10.1016/j.jns.2015.02.028. PMID 25725931.
- ↑ Pearce BD, Kruszon-Moran D, Jones JL (Aug 15, 2012). "The relationship between Toxoplasma gondii infection and mood disorders in the third National Health and Nutrition Survey". Biological Psychiatry. 72 (4): 290–5. doi:10.1016/j.biopsych.2012.01.003. PMID 22325983.
- ↑ Sugden, Karen; Moffitt, Terrie E.; Pinto, Lauriane; Poulton, Richie; Williams, Benjamin S.; Caspi, Avshalom; Tanowitz, Herbert B. (17 February 2016). "Is Toxoplasma Gondii Infection Related to Brain and Behavior Impairments in Humans? Evidence from a Population-Representative Birth Cohort". PLOS ONE. 11 (2): e0148435. doi:10.1371/journal.pone.0148435.
- 1 2 Torrey EF, Bartko JJ, Yolken RH (2012). "Toxoplasmosis gondii and other risk factors for Schizophrenia: An Update". Schizophrenia Bulletin. 38: 642–647. doi:10.1093/schbul/sbs043. PMID 22446566.
- 1 2 Arias, I; Sorlozano, A; Villegas, E; de Dios Luna, J; McKenney, K; Cervilla, J; Gutierrez, B; Gutierrez, J (April 2012). "Infectious agents associated with schizophrenia: a meta-analysis." (PDF). Schizophrenia Research. 136 (1-3): 128–36. doi:10.1016/j.schres.2011.10.026. PMID 22104141.
- 1 2 Flegr J (2013). "How and why toxoplasma makes us crazy". Trend in Parasitology. 29: 156–163. doi:10.1016/j.pt.2013.01.007.
- 1 2 Flegr J (Jan 1, 2013). "Influence of latent Toxoplasma infection on human personality, physiology and morphology: pros and cons of the Toxoplasma-human model in studying the manipulation hypothesis". The Journal of Experimental Biology. 216 (Pt 1): 127–33. doi:10.1242/jeb.073635. PMID 23225875.
- ↑ Torrey EF, Yolken RH (May 2007). "Schizophrenia and toxoplasmosis". Schizophrenia bulletin. 33 (3): 727–8. doi:10.1093/schbul/sbm026. PMC 2526129
 . PMID 17426051.
. PMID 17426051. - ↑ Stascheit F, Paul F, Harms L, Rosche B (2015). "Toxoplasma gondii Seropositivity Is Negatively Associated With Multiple Sclerosis". Journal of Neuroimmunology. 285: 119–124. doi:10.1016/j.jneuroim.2015.05.011.
- Parts of this article are taken from the public domain CDC factsheet: Toxoplasmosis
Bibliography
- Louis M Weiss; Kami Kim (28 April 2011). Toxoplasma Gondii: The Model Apicomplexan. Perspectives and Methods. Academic Press. ISBN 978-0-08-047501-1. Retrieved 12 March 2013.
- J. P. Dubey (15 April 2010). Toxoplasmosis of Animals and Humans, Second Edition. CRC Press. ISBN 978-1-4200-9237-0. Retrieved 12 March 2013.
- Dubey JP, Lindsay DS, Speer CA (April 1998). "Structures of Toxoplasma gondii Tachyzoites, Bradyzoites, and Sporozoites and Biology and Development of Tissue Cysts". Clinical Microbiology Reviews. 11 (2): 267–299. PMC 106833
 . PMID 9564564.
. PMID 9564564. - Jaroslav Flegr (2011). Pozor, Toxo!. Academia, Prague, Czech republic. ISBN 978-80-200-2022-2. Retrieved 2011. Check date values in:
|access-date=(help)
External links
- How a cat-borne parasite infects humans (National Geographic)
- Toxoplasmosis at Merck Manual of Diagnosis and Therapy Professional Edition
- Toxoplasmosis at Health Protection Agency (HPA), United Kingdom
- Pictures of Toxoplasmosis Medical Image Database
- Video-Interview with Professor Robert Sapolsky on Toxoplasmosis and its effect on human behavior (24:27 min)