Mechanotransduction
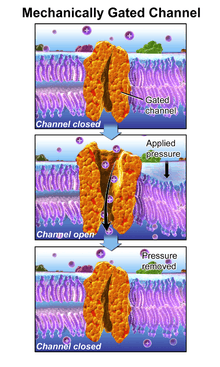
Mechanotransduction (mechano- + transduction) is any of various mechanisms by which cells convert mechanical stimulus into electrochemical activity.[1][2][3] This form of sensory transduction is responsible for a number of senses and physiological processes in the body, including proprioception, touch,[4] balance, and hearing.[5][6][7] The basic mechanism of mechanotransduction involves converting mechanical signals into electrical or chemical signals. In this process, a mechanically gated ion channel makes it possible for sound, pressure, or movement to cause a change in the excitability of specialized sensory cells and sensory neurons.[8] The stimulation of a mechanoreceptor causes mechanically sensitive ion channels to open and produce a transduction current that changes the membrane potential of the cell.[9] Typically the mechanical stimulus gets filtered in the conveying medium before reaching the site of mechanotransduction.[10] Cellular responses to mechanotransduction are variable and give rise to a variety of changes and sensations.
Ear
One such mechanism is the opening of ion channels in the hair cells of the cochlea in the inner ear.
Air pressure changes in the ear canal cause the vibrations of the tympanic membrane and middle ear ossicles. At the end of the ossicular chain, movement of the stapes footplate within the oval window of the cochlea, in turn, generates a pressure field within the cochlear fluids, imparting a pressure differential across the basilar membrane. A sinusoidal pressure wave results in localized vibrations of the organ of Corti: near the base for high frequencies, near the apex for low frequencies. The cochlea thus acts as an 'acoustic prism', distributing the energy of each Fourier component of a complex sound at different locations along its longitudinal axis. Hair cells in the cochlea are stimulated when the basilar membrane is driven up and down by differences in the fluid pressure between the scala vestibuli and scala tympani. Because this motion is accompanied by a shearing motion between the tectorial membrane and the reticular lamina of the organ of Corti, the hair bundles that link the two are deflected, which initiates mechano-electrical transduction. When the basilar membrane is driven upward, shear between the hair cells and the tectorial membrane deflects hair bundles in the excitatory direction, toward their tall edge. At the midpoint of an oscillation the hair bundles resume their resting position. When the basilar membrane moves downward, the hair bundles are driven in the inhibitory direction.
Basilar Membrane motion causes a shearing motion between the reticular lamina and the tectorial membrane, thereby activating the mechano-sensory apparatus of the hair bundle, which in turn generates a receptor potential in the hair cells.
Thus the sound pressure wave is transduced to an electrical signal which can be processed as sound in higher parts of the auditory system.
Skeletal Muscle
When a deformation is imposed on a muscle, changes in cellular and molecular conformations link the mechanical forces with biochemical signals, and the close integration of mechanical signals with electrical, metabolic, and hormonal signaling may disguise the aspect of the response that is specific to the mechanical forces.[11]
Cartilage
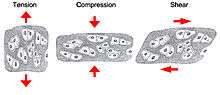
One of the main mechanical functions of articular cartilage is to act as a low-friction, load-bearing surface. Due to its unique location at joint surfaces, articular cartilage experiences a range of static and dynamic forces that include shear, compression and tension. These mechanical loads are absorbed by the cartilage extracellular matrix (ECM), where they are subsequently dissipated and transmitted to chondrocytes (cartilage cells).
Chondrocytes sense and convert the mechanical signals they receive into biochemical signals, which subsequently direct and mediate both anabolic (matrix building) and catabolic (matrix degrading) processes. These processes include the synthesis of matrix proteins (type II collagen and proteoglycans), proteases, protease inhibitors, transcription factors, cytokines and growth factors.[12][13]
The balance that is struck between anabolic and catabolic processes is strongly influenced by the type of loading that cartilage experiences. High strain rates (such as which occurs during impact loading) cause tissue damage, degradation, decreased matrix production and apoptosis.[14][15] Decreased mechanical loading over long periods, such as during extended bed-rest, causes a loss of matrix production.[16] Static loads have been shown to be detrimental to biosynthesis[17] while oscillatory loads at low frequencies (similar that of a normal walking gait) have been shown to be beneficial in maintaining health and increasing matrix synthesis.[18] Due to the complexity of in-vivo loading conditions and the interplay of other mechanical and biochemical factors, the question of what an optimal loading regimen may be or whether one exists remain unanswered.
Although studies have shown that, like most biological tissues, cartilage is capable of mechanotransduction, the precise mechanisms by which this is done remain unknown. However, there exist a few hypotheses which begin with the identification of mechanoreceptors.
In order for mechanical signals to be sensed, there need to be mechanoreceptors on the surface of chondrocytes. Candidates for chondrocyte mechanoreceptors include stretch-activated ion channels (SAC),[19] the hyaluronan receptor CD44, annexin V (a collagen type II receptor),[20] and integrin receptors (of which there exist several types on chondrocytes).
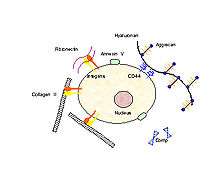
Using the integrin-linked mechanotransduction pathway as an example (being one of the better studied pathways), it has been shown to mediate chondrocyte adhesion to cartilage surfaces,[21] mediate survival signaling[22] and regulate matrix production and degradation.[23]
Integrin receptors have an extracellular domain that binds to the ECM proteins (collagen, fibronectin, laminin, vitronectin and osteopontin), and a cytoplasmic domain that interacts with intracellular signaling molecules. When an integrin receptor binds to its ECM ligand and is activated, additional integrins cluster around the activated site. In addition, kinases (e.g., focal adhesion kinase, FAK) and adapter proteins (e.g., paxillin, Pax, talin, Tal and Shc) are recruited to this cluster, which is called the focal adhesion complex (FAC). The activation of these FAC molecules in turn, triggers downstream events that up-regulate and /or down-regulate intracellular processes such as transcription factor activation and gene regulation resulting in apoptosis or differentiation.
In addition to binding to ECM ligands, integrins are also receptive to autocrine and paracrine signals such as growth factors in the TGF-beta family. Chondrocytes have been shown to secrete TGF-b, and upregulate TGF-b receptors in response to mechanical stimulation; this secretion may be a mechanism for autocrine signal amplification within the tissue.[24]
Integrin signaling is just one example of multiple pathways that are activated when cartilage is loaded. Some intracellular processes that have been observed to occur within these pathways include phosphorylation of ERK1/2, p38 MAPK, and SAPK/ERK kinase-1 (SEK-1) of the JNK pathway[25] as well as changes in cAMP levels, actin re-organization and changes in the expression of genes which regulate cartilage ECM content.[26]
More recent studies have hypothesized that chondrocyte primary cilium act as a mechanoreceptor for the cell, transducing forces from the extracellular matrix into the cell. Each chondrocyte has one cilium and it is hypothesized to transmit mechanical signals by way of bending in response to ECM loading. Integrins have been identified on the upper shaft of the cilium, acting as anchors to the collagen matrix around it.[27] Recent studies published by Wann et al. in FASEB Journal have demonstrated for the first time that primary cilia are required for chondrocyte mechanotransduction. Chondrocytes derived from IFT88 mutant mice did not express primary cilia and did not show the characteristic mechanosensitive up regulation of proteoglycan synthesis seen in wild type cells [28]
It is important to examine the mechanotransduction pathways in chondrocytes since mechanical loading conditions which represent an excessive or injuruous response upregulates synthetic activity and increases catabolic signalling cascades involving mediators such as NO and MMPs. In addition, studies by Chowdhury TT and Agarwal S have shown that mechanical loading which represents physiological loading conditions will block the production of catabolic mediators (iNOS, COX-2, NO, PGE2) induced by inflammatory cytokines (IL-1) and restore anabolic activities. Thus an improved understanding of the interplay of biomechanics and cell signalling will help to develop therapeutic methods for blocking catabolic components of the mechanotransduction pathway. A better understanding of the optimal levels of in vivo mechanical forces are therefore necessary for maintaining the health and viability of cartilage, preventative techniques may be devised for the prevention of cartilage degradation and disease.
References
- ↑ Biswas, Abhijit; Manivannan, M.; Srinivasan, Mandyam A. (2015). "Vibrotactile Sensitivity Threshold: Nonlinear Stochastic Mechanotransduction Model of the Pacinian Corpuscle". IEEE Transactions on Haptics. 8 (1): 102–113. doi:10.1109/TOH.2014.2369422. PMID 25398183.
- ↑ Katsumi, A.; Orr, AW; Tzima, E; Schwartz, MA (2003). "Integrins in Mechanotransduction". Journal of Biological Chemistry. 279 (13): 12001–4. doi:10.1074/jbc.R300038200. PMID 14960578.
- ↑ Qin, Y.; Qin, Y; Liu, J; Tanswell, AK; Post, M (1996). "Mechanical Strain Induces pp60src Activation and Translocation to Cytoskeleton in Fetal Rat Lung Cells". Journal of Biological Chemistry. 271 (12): 7066–71. doi:10.1074/jbc.271.12.7066. PMID 8636139.
- ↑ Biswas, Abhijit; Manivannan, M.; Srinivasan, Mandyam A. (2014). "Nonlinear two stage mechanotransduction model and neural response of Pacinian Corpuscle". Biomedical Science and Engineering Center Conference (BSEC), 2014 Annual Oak Ridge National Laboratory. USA: IEEE. pp. 1–4. doi:10.1109/BSEC.2014.6867740.
- ↑ Tavernarakis, Nektarios; Driscoll, Monica (1997). "Molecular Modeling of Mechanotransduction in the Nematodecaenorhabditis Elegans". Annual Review of Physiology. 59: 659–89. doi:10.1146/annurev.physiol.59.1.659. PMID 9074782.
- ↑ Howard, J; Roberts, W M; Hudspeth, A J (1988). "Mechanoelectrical Transduction by Hair Cells". Annual Review of Biophysics and Biophysical Chemistry. 17: 99–124. doi:10.1146/annurev.bb.17.060188.000531. PMID 3293600.
- ↑ Hackney, CM; Furness, DN (1995). "Mechanotransduction in vertebrate hair cells: Structure and function of the stereociliary bundle". The American journal of physiology. 268 (1 Pt 1): C1–13. PMID 7840137.
- ↑ Gillespie, Peter G.; Walker, Richard G. (2001). "Molecular basis of mechanosensory transduction". Nature. 413 (6852): 194–202. doi:10.1038/35093011. PMID 11557988.
- ↑ Grigg, P (1986). "Biophysical studies of mechanoreceptors". Journal of applied physiology. 60 (4): 1107–15. PMID 2422151.
- ↑ Biswas, Abhijit; Manivannan, M.; Srinivasan, Mandyam A. (2015). "Multiscale Layered Biomechanical Model of the Pacinian Corpuscle". IEEE Transactions on Haptics. 8 (1): 31–42. doi:10.1109/TOH.2014.2369416. PMID 25398182.
- ↑ Burkholder, TJ (2007). "Mechanotransduction in skeletal muscle". Frontiers in Bioscience. 12: 174–91. doi:10.2741/2057. PMC 2043154
 . PMID 17127292.
. PMID 17127292. - ↑ Fitzgerald, J. B.; Jin, M; Dean, D; Wood, DJ; Zheng, MH; Grodzinsky, AJ (2004). "Mechanical Compression of Cartilage Explants Induces Multiple Time-dependent Gene Expression Patterns and Involves Intracellular Calcium and Cyclic AMP". Journal of Biological Chemistry. 279 (19): 19502–11. doi:10.1074/jbc.M400437200. PMID 14960571.
- ↑ Fitzgerald, J. B.; Jin, M; Grodzinsky, AJ (2006). "Shear and Compression Differentially Regulate Clusters of Functionally Related Temporal Transcription Patterns in Cartilage Tissue". Journal of Biological Chemistry. 281 (34): 24095–103. doi:10.1074/jbc.M510858200. PMID 16782710.
- ↑ Kurz, Bodo; Jin, Moonsoo; Patwari, Parth; Cheng, Debbie M.; Lark, Michael W.; Grodzinsky, Alan J. (2001). "Biosynthetic response and mechanical properties of articular cartilage after injurious compression". Journal of Orthopaedic Research. 19 (6): 1140–6. doi:10.1016/S0736-0266(01)00033-X. PMID 11781016.
- ↑ Loening, A; James, IE; Levenston, ME; Badger, AM; Frank, EH; Kurz, B; Nuttall, ME; Hung, HH; Blake, SM (2000). "Injurious Mechanical Compression of Bovine Articular Cartilage Induces Chondrocyte Apoptosis". Archives of Biochemistry and Biophysics. 381 (2): 205–12. doi:10.1006/abbi.2000.1988. PMID 11032407.
- ↑ Behrens, Fred; Kraft, Ellen L.; Oegema, Theodore R. (1989). "Biochemical changes in articular cartilage after joint immobilization by casting or external fixation". Journal of Orthopaedic Research. 7 (3): 335–43. doi:10.1002/jor.1100070305. PMID 2703926.
- ↑ Torzilli, P. A.; Deng, X-H.; Ramcharan, M. (2006). "Effect of Compressive Strain on Cell Viability in Statically Loaded Articular Cartilage". Biomechanics and Modeling in Mechanobiology. 5 (2–3): 123–32. doi:10.1007/s10237-006-0030-5. PMID 16506016.
- ↑ Sah, Robert L.-Y.; Kim, Young-Jo; Doong, Joe-Yuan H.; Grodzinsky, Alan J.; Plass, Anna H. K.; Sandy, John D. (1989). "Biosynthetic response of cartilage explants to dynamic compression". Journal of Orthopaedic Research. 7 (5): 619–36. doi:10.1002/jor.1100070502. PMID 2760736.
- ↑ Mouw, J. K.; Imler, S. M.; Levenston, M. E. (2006). "Ion-channel Regulation of Chondrocyte Matrix Synthesis in 3D Culture Under Static and Dynamic Compression". Biomechanics and Modeling in Mechanobiology. 6 (1–2): 33–41. doi:10.1007/s10237-006-0034-1. PMID 16767453.
- ↑ Von Der Mark, K.; Mollenhauer, J. (1997). "Annexin V interactions with collagen". Cellular and Molecular Life Sciences (CMLS). 53 (6): 539. doi:10.1007/s000180050069.
- ↑ Kurtis, Melissa S.; Tu, Buu P.; Gaya, Omar A.; Mollenhauer, Jürgen; Knudson, Warren; Loeser, Richard F.; Knudson, Cheryl B.; Sah, Robert L. (2001). "Mechanisms of chondrocyte adhesion to cartilage: Role of β1-integrins, CD44, and annexin V". Journal of Orthopaedic Research. 19 (6): 1122–30. doi:10.1016/S0736-0266(01)00051-1. PMID 11781014.
- ↑ Pulai, Judit I.; Del Carlo, Marcello; Loeser, Richard F. (2002). "The ?5?1 integrin provides matrix survival signals for normal and osteoarthritic human articular chondrocytes in vitro". Arthritis & Rheumatism. 46 (6): 1528. doi:10.1002/art.10334.
- ↑ Millward–Sadler, S. J.; Wright, M. O.; Davies, L. W.; Nuki, G.; Salter, D. M. (2000). "Mechanotransduction via integrins and interleukin–4 results in altered aggrecan and matrix metalloproteinase 3 gene expression in normal, but not osteoarthritic, human articular chondrocytes". Arthritis & Rheumatism. 43 (9): 2091. doi:10.1002/1529-0131(200009)43:9<2091::AID-ANR21>3.0.CO;2-C.
- ↑ Millward-Sadler, S. J.; Salter, D. M. (2004). "Integrin-Dependent Signal Cascades in Chondrocyte Mechanotransduction". Annals of Biomedical Engineering. 32 (3): 435–46. doi:10.1023/B:ABME.0000017538.72511.48. PMID 15095818.
- ↑ Fanning, P. J.; Emkey, G; Smith, RJ; Grodzinsky, AJ; Szasz, N; Trippel, SB (2003). "Mechanical Regulation of Mitogen-activated Protein Kinase Signaling in Articular Cartilage". Journal of Biological Chemistry. 278 (51): 50940–8. doi:10.1074/jbc.M305107200. PMID 12952976.
- ↑ Urban, J. P. G. (1994). "The Chondrocyte: A Cell Under Pressure". Rheumatology. 33 (10): 901. doi:10.1093/rheumatology/33.10.901.
- ↑ McGlashan, S. R.; Jensen, CG; Poole, CA (2006). "Localization of Extracellular Matrix Receptors on the Chondrocyte Primary Cilium". Journal of Histochemistry and Cytochemistry. 54 (9): 1005–14. doi:10.1369/jhc.5A6866.2006. PMID 16651393.
- ↑ Wann AK, Zho N, Haycraft CJ, Jensen CG, Poole CA, McGlashen SR, Knight MM (2012) FASEB J. PMID 22223751
Further reading
- Khan, K M; Scott, A (2009). "Mechanotherapy: How physical therapists' prescription of exercise promotes tissue repair". British Journal of Sports Medicine. 43 (4): 247–52. doi:10.1136/bjsm.2008.054239. PMC 2662433
 . PMID 19244270.
. PMID 19244270. - Mammano, F.; Nobili, R (1993). "Biophysics of the cochlea: Linear approximation". The Journal of the Acoustical Society of America. 93 (6): 3320–32. Bibcode:1993ASAJ...93.3320M. doi:10.1121/1.405716. PMID 8326060.
- v. Békésy, Georg (1952). "DC Resting Potentials Inside the Cochlear Partition". The Journal of the Acoustical Society of America. 24: 72. Bibcode:1952ASAJ...24...72V. doi:10.1121/1.1906851.
- 1. Kandel, E.R., Schwartz, J.H., Jessell, T.M., Principles of Neural Science. New York: McGraw-Hill ed, ed. 4th. 2000.
- Hudspeth, A. J.; Choe, Y.; Mehta, A. D.; Martin, P. (2000). "Putting ion channels to work: Mechanoelectrical transduction, adaptation, and amplification by hair cells". Proceedings of the National Academy of Sciences. 97 (22): 11765. Bibcode:2000PNAS...9711765H. doi:10.1073/pnas.97.22.11765.
- Hudspeth, A. J. (1989). "How the ear's works work". Nature. 341 (6241): 397–404. Bibcode:1989Natur.341..397H. doi:10.1038/341397a0. PMID 2677742.
External links
- www.du.edu/~kinnamon/3640/hearing/hearing.html
- Cellular Mechanotransduction at the US National Library of Medicine Medical Subject Headings (MeSH)