Merkel-cell carcinoma
| Merkel-cell carcinoma | |
|---|---|
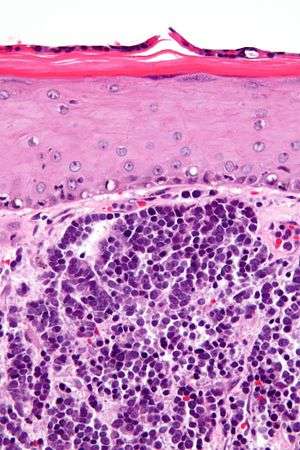 | |
| Micrograph of a Merkel-cell carcinoma. H&E stain. | |
| Classification and external resources | |
| Specialty | Oncology |
| ICD-10 | C44 (ILDS C44.L44) |
| ICD-9-CM | 209.31 - 209.36 |
| ICD-O | M8247/3 |
| DiseasesDB | 31386 |
| eMedicine | DERM/262 ent/714 |
| MeSH | D015266 |
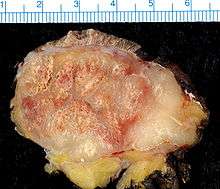
Merkel-cell carcinoma (MCC) is a rare and highly aggressive skin cancer, which, in most cases, is caused by the Merkel cell polyomavirus (MCV) discovered by scientists at the University of Pittsburgh in 2008.[1] It is also known as cutaneous APUDoma, primary neuroendocrine carcinoma of the skin, primary small cell carcinoma of the skin, and trabecular carcinoma of the skin.[2]
Approximately 80% of Merkel-cell carcinomas are caused by MCV. The virus is clonally integrated into the cancerous Merkel cells. In addition, the virus has a particular mutation only when found in cancer cells, but not when it is detected in healthy skin cells.[3] Direct evidence for this oncogenetic mechanism comes from research showing that inhibition of production of MCV proteins causes MCV-infected Merkel carcinoma cells to die but has no effect on malignant Merkel cells that are not infected with this virus.[4][5] MCV-uninfected tumors, which account for approximately 20% of Merkel-cell carcinomas, appear to have a separate and as-yet unknown cause.[6] These tumors tend to have extremely high genome mutation rates, due to ultraviolet light exposure, whereas MCV-infected Merkel cell carcinomas have low rates of genome mutation.[7] No other cancers have been confirmed so far to be caused by this virus. Because of the viral origin for this cancer, immunotherapies are a promising avenue for research to treat virus-positive Merkel-cell carcinoma.
This cancer is considered to be a form of neuroendocrine tumor. While patients with a small tumor (less than 2 cm) that has not yet metastasized to regional lymph nodes have an expected 5-year survival rate of more than 80 percent, once a lesion has metastasized regionally, the rate drops to about 50 percent. Up to half of patients that have been seemingly treated successfully (i.e. that initially appear cancer-free) subsequently suffer a recurrence of their disease.[8] Recent reviews cite an overall 5-year survival rate of about 60% for all MCC combined.[6]
Merkel-cell carcinoma occurs most often on the sun-exposed face, head, and neck.[9]
Signs and symptoms
MCC usually presents as a firm, painless, nodule (up to 2 cm diameter) or mass (>2 cm diameter). These flesh-colored, red, or blue tumors typically vary in size from 0.5 cm (less than one-quarter of an inch) to more than 5 cm (2 inches) in diameter, and usually enlarge rapidly. Although MCC's may arise almost anywhere on the body, about half originate on sun-exposed areas of the head and neck, one-third on the legs, and about one-sixth on the arms. In about 12% of cases, no obvious anatomical site of origin ("primary site") can be identified.[10]
Merkel-cell cancers tend to invade locally, infiltrating the underlying subcutaneous fat, fascia, and muscle, and typically metastasize early in their natural history, most often to the regional lymph nodes. MCCs also spread aggressively through the blood vessels, particularly to liver, lung, brain, and bone.
Cause
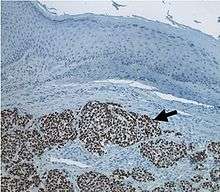
A newly discovered virus called Merkel cell polyomavirus (MCV) likely contributes to the development of the majority of MCC.[1][11] Approximately 80% of MCC have this virus integrated in a monoclonal pattern,[12][13] indicating that the infection was present in a precursor cell before it became cancerous. At least 20% of MCC tumors are not infected with MCV, suggesting that MCC may have other causes as well.
Polyomaviruses have been known to be oncogenic (cancer-causing) viruses in animals since the 1950s,[14] but MCV is the first polyomavirus strongly suspected to cause tumors in humans. Like other tumor viruses, most people who are infected with MCV probably do not develop MCC. It is currently unknown what other steps or co-factors are required for MCC-type cancers to develop.[15] Exposure to ultraviolet (UV) light, which is found in natural sunlight and in the artificial light generated by tanning beds, probably contributes to MCC development in a high percentage of cases. MCC can also occur together with other sun exposure-related skin cancers that are not infected with MCV (i.e. basal cell carcinoma, squamous cell carcinoma, melanoma). Intriguingly, most MCV viruses obtained so far from tumors have specific mutations that render the virus uninfectious.[16][17] It is unknown whether these particular mutations result from sun exposure. MCC also occurs more frequently than would otherwise be expected among immunosuppressed patients, such as transplant patients, AIDS patients, and the elderly persons, suggesting that the initiation and progression of the disease is modulated by the immune system.[18]
While infection with MCV is common in humans,[19] MCC patients whose tumors contain MCV have higher antibody levels against the virus than similarly infected healthy adults.[20] A recent study of a large patient registry from Finland suggests that individuals with MCV-positive MCC's have better prognoses than do MCC patients without MCV infection.[21] While MCV-positive MCC may be a less aggressive form of the disease, the results of the aforementioned study may instead be due to significant differences in other confounding factors, including tumor stage at the time of diagnosis, the age of the patient, or the location of the tumor rather than any intrinsic difference in disease aggressiveness or response to therapy.
Epidemiology
This type of cancer occurs most often in Caucasians between 60 and 80 years of age, and its rate of incidence is about twice as high in males as in females. There are roughly 1,500 new cases of MCC diagnosed each year in the United States,[6] as compared to around 60,000 new cases of melanoma and over 1 million new cases of nonmelanoma skin cancer.[22] MCC is sometimes mistaken for other histological types of cancer, including basal cell carcinoma, squamous cell carcinoma, malignant melanoma, lymphoma, and small cell carcinoma, or as a benign cyst. Researchers believe that exposure to sunlight or ultraviolet light (such as in a tanning bed) may increase the risk of developing this disease. Similar to melanoma, the incidence of MCC in the US is increasing rapidly.[6]
Immunosuppression can profoundly increase the odds of developing Merkel-cell carcinoma. Merkel-cell carcinoma occurs 30 times more often in people with chronic lymphocytic leukemia and 13.4 times more often in people with advanced HIV as compared to the general population; solid organ transplant recipients have a 10-fold increased risk compared to the general population.[9]
Treatment
Since Merkel-cell cancer is uncommon[6] and difficult to diagnose, patients may want a second opinion about the diagnosis and treatment plan before starting treatment. However, early diagnosis and treatment of Merkel-cell cancers are important factors in decreasing the chance of metastasis, after which it is exceptionally difficult to cure.
The number of studies focusing on the development of new targeted anticancer therapy is steadily rising, and thus there is hope that new drug regimes for patients with distant and systemic Merkel-cell carcinoma disease will be available in the near future. In particular, many study groups are looking for new strategies to target the MCV either to prevent infection or to inhibit viral-induced carcinogenesis.[23]
Even highly advanced metastatic Merkel cell carcinoma can be responsive to PD-1 inhibitor treatment,[24] providing promise for new chemotherapeutic and immunotherapeutic options.
Surgery
Surgery is usually the first treatment that a patient undergoes for Merkel-cell cancer. Lesions usually appear purple-red in color, and there is little else to distinguish this variant of skin cancer from other types. Its identity usually comes as a surprise after surgery and pathologic examination.
As with surgery for most other forms of cancer, it is normal for the surgeon to remove a border of healthy tissue surrounding the tumor. While it has been thought that leaving this margin may not be as critical as it is in the surgical resection of melanoma, studies also reveal that local recurrences are fairly common in MCC near the site of the surgery.
Local or regional lymph nodes are usually removed if the lesion is more than 1 cm in diameter, due to a high risk that they will contain cancer cells (micrometastasis) that could develop into a new tumor or spread further. Sometimes, however, the doctor will first perform a "sentinel lymph node biopsy". In this procedure, the doctor injects a dye or radioactive substance near the tumor. This material flows into adjacent lymph nodes, which are identified, removed, and checked for cancer cells, indicating the sites where cancer is most likely to spread (the "sentinel" nodes). This procedure has been demonstrated to be an important prognostic indicator. Results help dictate the use of appropriate adjuvant therapies. Usually, however, surgery alone is insufficient to control Merkel-cell carcinoma.
Radiation and chemotherapy
Radiotherapy is commonly used to treat Merkel-cell cancers. The radiotherapy fields used are usually very large so as to cover sufficient areas of skin. This is necessary because of MCC's aggressive local and regional metastatic behavior.
Adjuvant radiotherapy has been shown to be effective in reducing the rates of recurrence and in increasing the survival of patients with MCC.[9] Patients who present with no distant metastases and a negative sentinel lymph node biopsy have a very good prognosis when treated with both surgery and radiotherapy (approximately 90% survival rate at five years).
Metastatic MCC may respond to treatment with chemotherapy and/or radiation, but current multimodal therapies are usually not curative. Intensive treatment can be effective in shrinking the tumor and improving operability when tumors are too large to be removed or located in a place where removal would be difficult or dangerous, or in palliation of signs and symptoms caused by metastatic tumors.
Sentinel lymph node biopsy
Sentinel lymph node biopsy (SLNB) detects MCC spread in one third of patients whose tumors would have otherwise been clinically and radiologically understaged, and who may not have received treatment to the involved node bed. There was a significant benefit of adjuvant nodal therapy, but only when the SLNB was positive. Thus, SLNB is important for both prognosis and therapy and should be performed routinely for patients with MCC. In contrast, computed tomographic scans have poor sensitivity in detecting nodal disease as well as poor specificity in detecting distant disease.[25]
Notable people who have had Merkel-cell carcinoma
- Avigdor Arikha – Paris-based painter and art historian
- David Brudnoy – Boston talk radio host
- Stan Collender - Executive vice president at MSLGROUP, a global communications agency.[26]
- Susan Manber - Chief Planning Officer at DigitasHealth LifeBrands, a global health and wellness communications agency
- Al Copeland – New Orleans entrepreneur, powerboat racer
- Al Davis – Principal owner of the Oakland Raiders of the National Football League
- Ed Derwinski – U.S. Representative from Illinois and 1st Secretary of Veterans Affairs
- Leonard Hirshan – longtime manager of Clint Eastwood who also counted Edward G. Robinson, Greer Garson, Sophia Loren, Angela Lansbury, Sammy Davis Jr. and Bruce Beresford among his clients over the course of his six-decade career as an agent and manager, died January 31, 2014 in Beverly Hills of Merkel-cell carcinoma.[27]
- Lawrence Larsen
- Max Perutz – Nobel Prize–winning chemist
- Lindsay Thompson – Former Premier of Victoria, Australia
- Joe Zawinul – Jazz-fusion pioneer
- John Fitch – Famous race car driver and road safety pioneer
- Carl Mundy – 30th Commandant of the United States Marine Corps
- Geoffrey Penwill Parsons AO OBE (15 June 1929 – 26 January 1995), the Australian accompanist to singers and instrumentalists, considered one of the world's finest and most sympathetic accompanist of lieder singers.
References
- 1 2 Feng, H.; Shuda, M.; Chang, Y.; Moore, P. S. (2008). "Clonal Integration of a Polyomavirus in Human Merkel Cell Carcinoma". Science. 319 (5866): 1096–100. doi:10.1126/science.1152586. PMC 2740911
 . PMID 18202256.
. PMID 18202256. - ↑ Rapini, Ronald P.; Bolognia, Jean L.; Jorizzo, Joseph L. (2007). Dermatology: 2-Volume Set. St. Louis: Mosby. ISBN 1-4160-2999-0.
- ↑ Shuda M, Feng H, Kwun HJ; et al. (2008). "T antigen mutations are a human tumor-specific signature for Merkel cell polyomavirus.". Proc Natl Acad Sci U S A. 105 (42): 16272–7. doi:10.1073/pnas.0806526105. PMC 2551627
 . PMID 18812503.
. PMID 18812503. - ↑ Houben R, Shuda M, Weinkam R, Schrama D, Feng H, Chang Y, et al. (2010). "Merkel cell polyomavirus infected Merkel cell carcinoma cells require expression of viral T antigens". J Virol. 84 (14): 7064–72. doi:10.1128/jvi.02400-09.
- ↑ Shuda M, Kwun HJ, Feng H, Chang Y, Moore PS (2011). "Human Merkel cell polyomavirus small T antigen is an oncoprotein targeting the 4E-BP1 translation regulator". J Clin Invest. 121 (9): 3623–34. doi:10.1172/jci46323.
- 1 2 3 4 5 Schrama, D.; Ugurel, S.; Becker, J. R. C. (2012). "Merkel cell carcinoma". Current Opinion in Oncology. 24 (2): 141–149. doi:10.1097/CCO.0b013e32834fc9fe. PMID 22234254.
- ↑ Wong, Stephen Q.; Waldeck, Kelly; Vergara, Ismael A.; Schröder, Jan; Madore, Jason; Wilmott, James S.; Colebatch, Andrew J.; De Paoli-Iseppi, Ricardo; Li, Jason (2015-12-15). "UV-Associated Mutations Underlie the Etiology of MCV-Negative Merkel Cell Carcinomas". Cancer Research. 75 (24): 5228–5234. doi:10.1158/0008-5472.CAN-15-1877. ISSN 1538-7445. PMID 26627015.
- ↑ Allen PJ, Bowne WB, Jaques DP, Brennan MF, Busam K, Coit DG (2005). "Merkel cell carcinoma: prognosis and treatment of patients from a single institution". J. Clin. Oncol. 23 (10): 2300–9. doi:10.1200/JCO.2005.02.329. PMID 15800320.
- 1 2 3 Hasan S, Triplet J, Li Z, Mansur D (November 2013). "The Role of Postoperative Radiation and Chemoradiation in Merkel Cell Carcinoma: A Systematic Review of the Literature". Front Oncol. 3: 276. doi:10.3389/fonc.2013.00276. PMID 24294591.
- ↑ Deneve JL, Messina JL, Marzban SS et al. Merkel Cell Carcinoma of Unknown Primary Origin. Ann Surg Oncol 2012 Jan 21.
- ↑
- ↑
- ↑
- ↑
- ↑ "New virus linked to rare but lethal skin cancer". The Age. Retrieved 2008-02-26.
- ↑
- ↑
- ↑ http://www3.interscience.wiley.com/cgi-bin/fulltext/114266344/HTMLSTART]
- ↑
- ↑
- ↑
- ↑ Hodgson NC (2005). "Merkel cell carcinoma: changing incidence trends". J Surg Oncol. 89 (1): 1–4. doi:10.1002/jso.20167. PMID 15611998.
- ↑ Munde, Prashant (17 Jan 2014). "Pathophysiology of merkel cell". Journal of Oral and Maxillofacial Pathology. 17 (3): 408–412. doi:10.4103/0973-029x.125208.
- ↑ Nghiem, Paul T.; Bhatia, Shailender; Lipson, Evan J.; Kudchadkar, Ragini R.; Miller, Natalie J.; Annamalai, Lakshmanan; Berry, Sneha; Chartash, Elliot K.; Daud, Adil (2016-04-19). "PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma". The New England Journal of Medicine. 374: 2542–52. doi:10.1056/NEJMoa1603702. ISSN 1533-4406. PMC 4927341
 . PMID 27093365.
. PMID 27093365. - ↑ Gupta SG, Wang LC, Peñas PF, Gellenthin M, Lee SJ, Nghiem P (2006). "Sentinel lymph node biopsy for evaluation and treatment of patients with Merkel cell carcinoma: The Dana-Farber experience and meta-analysis of the literature". Arch Dermatol. 142 (6): 685–90. doi:10.1001/archderm.142.6.685. PMID 16785370.
- ↑ Collender, Stan. "Clinical Trials Need Cancer Patients". The New York Times. Retrieved 2015-06-19.
- ↑ Colker, David (2014-02-06). "Leonard Hirshan dies at 86; longtime agent for Clint Eastwood". The Los Angeles Times. Retrieved 2015-06-28.
External links
- Merkel Cell Cancer Discussion Group
- "Merkel cell carcinoma--Information for Patients & Their Physicians". Seattle Cancer Care Alliance. 2008-03-25. Retrieved 2008-04-13.
- National Cancer Institute. "Merkel Cell Carcinoma". National Institutes of Health (US). Retrieved 2011-01-20.