Prenatal development
Prenatal or antenatal development is the process in which a human embryo and later fetus (or foetus) develops during pregnancy, from fertilization until birth. Often, the terms fetal development, or embryology are used in a similar sense.
After fertilization, the process of embryogenesis (the early stages of prenatal development) begins. By the end of the tenth week of gestational age the embryo has acquired its basic form and the next period is that of fetal development where the organs become fully developed. This fetal period is described both topically (by organ) and chronologically (by time) with major occurrences being listed by gestational age.
Definitions of periods

- The perinatal period (from Greek peri, "about, around" and Latin nasci "to be born") is "around the time of birth". In developed countries and at facilities where expert neonatal care is available, it is considered from 22 completed weeks (154 days) of gestation (the time when birth weight is normally 500 g) to 7 completed days after birth.[1] In many developing countries, the starting point of this period is considered 28 completed weeks of gestation (or weight more than 1000 g).[2] In ICD-10, a medical classification list by the WHO, there is a particular chapter relating to certain conditions originating in the perinatal period.
- The antepartum period (from Latin ante "before" and parere "to give birth") is literally equivalent to prenatal (from Latin pre- "before" and nasci "to be born"). Practically, however, antepartum usually refers to the period between the 24th/26th week of gestational age until birth, for example in antepartum hemorrhage.[3][4]
Fertilization
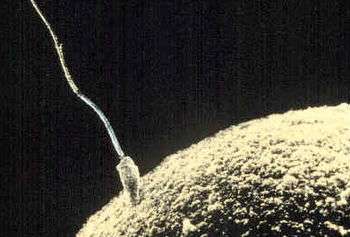
When semen is released into the vagina, the spermatozoa travel through the cervix and body of the uterus and into the Fallopian tubes. Fertilization of the egg cell (ovum), usually takes place in one of the Fallopian tubes. Many sperm are released with the possibility of just one sperm cell managing to adhere to and enter the thick protective shell-like layer surrounding the ovum. The first sperm that penetrates fully into the egg donates its genetic material (DNA). The egg then polarizes, repelling any additional sperm. The resulting combination is called a zygote, a new and genetically unique organism. The term "conception" refers variably to either fertilization or to formation of the conceptus after its implantation in the uterus, and this terminology is controversial.
Prior to fertilization, each ovum, as a gamete, contains half of the genetic material that will fuse with the male gamete, which carries the other half of the genetic material (DNA). The ovum only carries the X female sex chromosome whilst the sperm carries a single sex chromosome of either an X or a male Y chromosome. The resulting human zygote is similar to the majority of somatic cells because it contains two copies of the genome in a diploid set of chromosomes. One set of chromosomes came from the nucleus of the ovum and the second set from the nucleus of the sperm.
The zygote is male if the egg is fertilized by a sperm that carries a Y chromosome, and it is female if the egg is fertilized by a sperm that carries an X chromosome.[5] The Y chromosome contains a gene, SRY, which will switch on androgen production at a later stage, leading to the development of a male body type. In contrast, the mitochondrial genetic information of the zygote comes entirely from the mother via the ovum.
Embryonic period
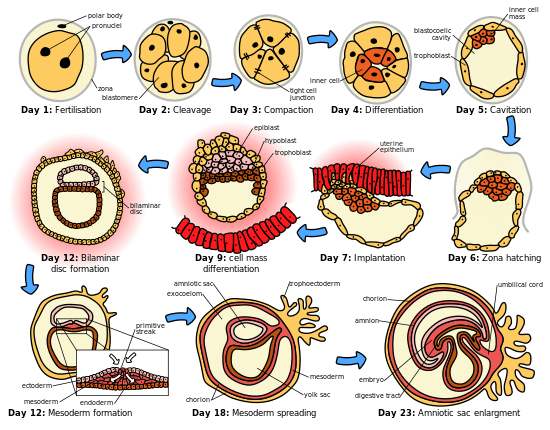
The embryonic period in humans begins at fertilization (penetration of the egg by the sperm) and continues until the end of the 10th week of gestation (8th week by embryonic age). The period of two weeks from fertilization is also referred to as the germinal stage.
The embryo spends the next few days traveling down the Fallopian tube. It starts out as a single cell zygote and then divides several times to form a ball of cells called a morula. Further cellular division is accompanied by the formation of a small cavity between the cells. This stage is called a blastocyst. Up to this point there is no growth in the overall size of the embryo, as it is confined within a glycoprotein shell, known as the zona pellucida. Instead, each division produces successively smaller cells.
The blastocyst reaches the uterus at roughly the fifth day after fertilization. It is here that lysis of the zona pellucida occurs. This process is analogous to zona hatching, a term that refers to the emergence of the blastocyst from the zona pellucida, when incubated in vitro. This allows the trophectoderm cells of the blastocyst to come into contact with, and adhere to, the endometrial cells of the uterus. The trophectoderm will eventually give rise to extra-embryonic structures, such as the placenta and the membranes. The embryo becomes embedded in the endometrium in a process called implantation. In most successful pregnancies, the embryo implants 8 to 10 days after ovulation.[6] The embryo, the extra-embryonic membranes, and the placenta are collectively referred to as a conceptus, or the "products of conception".
Rapid growth occurs and the embryo's main features begin to take form. This process is called differentiation, which produces the varied cell types (such as blood cells, kidney cells, and nerve cells). A spontaneous abortion, or miscarriage, in the first trimester of pregnancy is usually[7] due to major genetic mistakes or abnormalities in the developing embryo. During this critical period (most of the first trimester), the developing embryo is also susceptible to toxic exposures, such as:
- Alcohol, certain drugs, and other toxins that cause birth defects, such as fetal alcohol syndrome
- Infection (such as rubella or cytomegalovirus)
- Radiation from x-rays or radiation therapy
- Nutritional deficiencies such as lack of folate which contributes to spina bifida
Changes by weeks of gestation
Gestational age vs. embryonic age
Gestational age is the time that has passed since the onset of the last menstruation, which generally or as standard occurs 2 weeks before the actual fertilization. Embryonic age, in contrast measures the actual age of the embryo or fetus from the time of fertilization. Nevertheless, menstruation has historically been the only means of estimating embryonal/fetal age, and is still the presumed measure if not else specified. However, the actual duration between last menstruation and fertilization may in fact differ from the standard 2 weeks by several days.
Thus, the first week of embryonic age is already week three counting with gestational age.
Furthermore, the number of the week is one more than the actual age of the embryo/fetus. For example, the embryo is 0 whole weeks old during the 1st week after fertilization.
The following table summarizes the various expression systems during week number x of gestation.
| Week number | Initial age (whole weeks) | |
| Gestational | x | x-1 |
| Embryonic | x-2 | x-3 |
Week 3
Gestational age: 2 weeks and 0 days until 2 weeks and 6 days old. 15–21 days from last menstruation.
Embryonic age: Week nr 1. 0 (whole) weeks old. 1–7 days from fertilization.
- Fertilization of the ovum to form a new human organism, the human zygote. (day 1 of fertilization[8])
- The zygote undergoes mitotic cellular divisions, but does not increase in size. This mitosis is also known as cleavage. A hollow cavity forms marking the blastocyst stage. (day 1.5–3 of fert.[8])
- The blastocyst contains only a thin rim of trophoblast cells and a clump of cells at one end known as the "embryonic pole" which include embryonic stem cells.
- The embryo hatches from its protein shell (zona pellucida) and performs implantation onto the endometrial lining of the mother's uterus. (day 5–6 of fert.[8])
- If separation into identical twins occurs, 1/3 of the time it will happen before day 5.[9]
Week 4
Gestational age: 3 weeks and 0 days until 3 weeks and 6 days old. 22–28 days from last menstruation.
Embryonic age: Week nr 2. 1 week old. 8–14 days from fertilization.
- Trophoblast cells surrounding the embryonic cells proliferate and invade deeper into the uterine lining. They will eventually form the placenta and embryonic membranes. The blastocyst is fully implanted day 7–12 of fert.[8]
- Formation of the yolk sac.
- The embryonic cells flatten into a disk, two cells thick.
- If separation into identical twins occurs, 2/3 of the time it will happen between days 5 and 9. If it happens after day 9, there is a significant risk of the twins being conjoined.
- Primitive streak develops. (day 13 of fert).[8]
- Primary stem villi appear. (day 13 of fert).[8]
Week 5
Gestational age: 4 weeks and 0 days until 4 weeks and 6 days old. 29–35 days from last menstruation.
Embryonic age: Week nr 3. 2 weeks old. 15–21 days from fertilization.
- A notochord forms in the center of the embryonic disk. (day 16 of fert.[8])
- Gastrulation commences. (day 16 of fert.[8])
- A neural groove (future spinal cord) forms over the notochord with a brain bulge at one end. Neuromeres appear. (day 18 of fert.[8])
- Somites, the divisions of the future vertebra, form. (day 20 of fert.[8])
- Primitive heart tube is forming. Vasculature begins to develop in embryonic disc. (day 20 of fert.[8])

Week 6
Gestational age: 5 weeks and 0 days until 5 weeks and 6 days old. 36–42 days from last menstruation.
Embryonic age: Week nr 4. 3 weeks old. 22–28 days from fertilization.
- The embryo measures 4 mm (1/8 inch) in length and begins to curve into a C shape.
- The heart bulges, further develops, and begins to beat in a regular rhythm. Septum primum appears.[8]
- Pharyngeal arches, grooves which will form structures of the face and neck, form.
- The neural tube closes.
- The ears begin to form as otic pits.
- Arm buds and a tail are visible.
- Lung bud, the first traits of the lung appear.[8]
- Hepatic plate, the first traits of the liver appear.[8]
- Buccopharyngeal membrane ruptures. This is the future mouth.[8]
- Cystic diverticulum, which will become the gallbladder, and dorsal pancreatic bud, which will become the pancreas appear.[8]
- Urorectal septum begins to form. Thus, the rectal and urinary passageways become separated.[8]
- Anterior and posterior horns differentiate in the spinal cord.[8]
- Spleen appears.[8]
- Ureteric buds appear.[8]
Week 7
Gestational age: 6 weeks and 0 days until 6 weeks and 6 days old. 43–49 days from last menstruation.
Embryonic age: Week nr 5. 4 weeks old. 29–35 days from fertilization.
- The embryo measures 1.6 cm (5/4 inch) in length and weighs about 1 gram.[11]
- Optic vesicles and optic cups form the start of the developing eye.
- Nasal pits form.
- The brain divides into 5 vesicles, including the early telencephalon.
- Leg buds form and hands form as flat paddles on the arms.
- Rudimentary blood moves through primitive vessels connecting to the yolk sac and chorionic membranes.
- The metanephros, precursor of the definitive kidney, starts to develop.
- The initial stomach differentiation begins.

Week 8
Gestational age: 7 weeks and 0 days until 7 weeks and 6 days old. 50–56 days from last menstruation.
Embryonic age: Week nr 6. 5 weeks old. 36–42 days from fertilization.
- The embryo measures 13 mm (1/2 inch) in length.
- Lungs begin to form.
- The brain continues to develop.
- Arms and legs have lengthened with foot and hand areas distinguishable.
- The hands and feet have digits, but may still be webbed.
- The gonadal ridge begins to be perceptible.
- The lymphatic system begins to develop.
- Main development of sex organs starts.
Week 9
Gestational age: 8 weeks and 0 days until 8 weeks and 6 days old. 57–63 days from last menstruation.
Embryonic age: Week nr 7. 6 weeks old. 43–49 days from fertilization.
- The embryo measures 18 mm (3/4 inch) in length.
- Fetal heart tone (the sound of the heart beat) can be heard using doppler.
- Nipples and hair follicles begin to form.
- Location of the elbows and toes are visible.
- Spontaneous limb movements may be detected by ultrasound.
- All essential organs have at least begun.
- The vitelline duct normally closes.
Fetal period
From the 10th week of gestation (8th week of development), the developing organism is called a fetus.
All major structures are already formed in the fetus, but they continue to grow and develop.
Since the precursors of all the major organs are created by this time, the fetal period is described both by organ and by a list of changes by weeks of gestational age.
Because the precursors of the organs are now formed, the fetus is not as sensitive to damage from environmental exposure as the embryo was. Instead, toxic exposure often causes physiological abnormalities or minor congenital malformation.
Changes by organ
Each organ has its own development.
- Development of circulatory system
- Development of digestive system
- Development of the endocrine system
- Development of integumentary system
- Development of lymphatic system
- Development of muscular system
- Development of nervous system
- Development of the urinary and reproductive system
- Development of respiratory system
Changes by weeks of gestation
Weeks 10 to 12
Gestational age: 9 weeks and 0 days until 11 weeks and 6 days old.
Embryonic age: 7 weeks and 0 days until 9 weeks and 6 days old.

- Embryo measures 30–80 mm (1.2–3.2 inches) in length.
- Ventral and dorsal pancreatic buds fuse during the 8th week
- Intestines rotate.
- Facial features continue to develop.
- The eyelids are more developed.
- The external features of the ear begin to take their final shape.
- The head comprises nearly half of the fetus' size.
- The face is well formed.
- The eyelids close and will not reopen until about the 28th week.
- Tooth buds, which will form the baby teeth, appear.
- The limbs are long and thin.
- The fetus can make a fist with its fingers.
- Genitals appear well differentiated.
- Red blood cells are produced in the liver.
- Heartbeat can be detected by ultrasound.[14]
Weeks 13 to 16
Gestational age: 12 weeks and 0 days until 15 weeks and 6 days old.
Embryonic age: 10 weeks and 0 days until 13 weeks and 6 days old.
- The fetus reaches a length of about 15 cm (6 inches).
- A fine hair called lanugo develops on the head.
- Fetal skin is almost transparent.
- More muscle tissue and bones have developed, and the bones become harder.
- The fetus makes active movements.
- Sucking motions are made with the mouth.
- Meconium is made in the intestinal tract.
- The liver and pancreas produce fluid secretions.
- From week 13, sex prediction by obstetric ultrasonography is almost 100% accurate.[15]
- At week 15, main development of external genitalia is finished.

Week 21
Gestational age: 20 weeks old.
Embryonic age: 18 weeks old.
- The fetus reaches a length of 20 cm (8 inches).
- Lanugo covers the entire body.
- Eyebrows and eyelashes appear.
- Nails appear on fingers and toes.
- The fetus is more active with increased muscle development.
- "Quickening" usually occurs (the mother and others can feel the fetus moving).
- The fetal heartbeat can be heard with a stethoscope.
Week 23
Gestational age: 22 weeks old.
Embryonic age: 20 weeks old.
- The fetus reaches a length of 28 cm (11.2 inches).
- The fetus weighs about 500g.
- Eyebrows and eyelashes are well formed.
- All of the eye components are developed.
- The fetus has a hand and startle reflex.
- Footprints and fingerprints continue forming.
- Alveoli (air sacs) are forming in lungs.
Week 26
Gestational age: 24 weeks old.
Embryonic age: Week nr 25. 24 weeks old.
- The fetus reaches a length of 38 cm (15 inches).
- The fetus weighs about 1.2 kg (2 lb 11 oz).
- The brain develops rapidly.
- The nervous system develops enough to control some body functions.
- The eyelids open and close.
- The cochleae are now developed, though the myelin sheaths in neural portion of the auditory system will continue to develop until 18 months after birth.
- The respiratory system, while immature, has developed to the point where gas exchange is possible.
Week 31
Gestational age: 30 weeks old.
Embryonic age: Week nr 29. 28 weeks old.
- The fetus reaches a length of about 38–43 cm (15–17 inches).
- The fetus weighs about 1.5 kg (3 lb 0 oz).
- The amount of body fat rapidly increases.
- Rhythmic breathing movements occur, but lungs are not fully mature.
- Thalamic brain connections, which mediate sensory input, form.
- Bones are fully developed, but are still soft and pliable.
- The fetus begins storing a lot of iron, calcium and phosphorus.
Week 35
Gestational age: 34 weeks old.
Embryonic age: Week nr 33. 32 weeks old.
- The fetus reaches a length of about 40–48 cm (16–19 inches).
- The fetus weighs about 2.5 to 3 kg (5 lb 12 oz to 6 lb 12 oz).
- Lanugo begins to disappear.
- Body fat increases.
- Fingernails reach the end of the fingertips.
- A baby born at 36 weeks has a high chance of survival, but may require medical interventions.

Weeks 36 to 40
Gestational age: 35 and 0 days until 39 weeks and 6 days old.
Embryonic age: Weeks nr 34–38. 33–37 weeks old.
- The fetus is considered full-term at the end of the 39th week of gestational age.
- It may be 48 to 53 cm (19 to 21 inches) in length.
- The lanugo is gone except on the upper arms and shoulders.
- Fingernails extend beyond fingertips.
- Small breast buds are present on both sexes.
- Head hair is now coarse and thickest.
The development is continued postnatally with adaptation to extrauterine life and child development stages.
Nutrition
The fetus passes through 3 phases of acquisition of nutrition from mother:[18]
- Absorption phase: Zygote is nourished by cellular cytoplasm and secretions in fallopian tubes and uterine cavity.
- Histoplasmic transfer: After nidation and before establishment of uteroplacental circulation, fetal nutrition is derived from decidual cells and maternal blood pools that open up as a result of eroding activity of trophoblasts.
- Hematotrophic phase: After third week of gestation, substances are transported passively via intervillous space.
Growth rate
Growth rate of fetus is linear up to 37 weeks of gestation, after which it plateaus.[18] The growth rate of an embryo and infant can be reflected as the weight per gestational age, and is often given as the weight put in relation to what would be expected by the gestational age. A baby born within the normal range of weight for that gestational age is known as appropriate for gestational age (AGA). An abnormally slow growth rate results in the infant being small for gestational age, and, on the other hand, an abnormally large growth rate results in the infant being large for gestational age. A slow growth rate and preterm birth are the two factors that can cause a low birth weight. Low birth weight (below 2000 grams) can ultimately increase the likelihood of schizophrenia by almost four times. [19]
The growth rate can be roughly correlated with the fundal height which can be estimated by abdominal palpation. More exact measurements can be performed with obstetric ultrasonography.
Factors influencing growth rate
Intrauterine growth restriction is one of the causes of low birth weight associated with over half of neonatal deaths.[20]
- Poverty
Poverty has been linked to poor prenatal care and has been an influence on prenatal development. Women in poverty are more likely to have children at a younger age, which results in low birth weight. Many of these expecting mothers have little education and are therefore less aware of the risks of smoking, drinking alcohol, and drug use - other factors that influence the growth rate of a fetus. Women in poverty are more likely to have diseases that are harmful to the fetus.
- Mother's age
Women between the ages of 16 and 35 have a healthier environment for a fetus than women under 16 or over 35. Women between this age gap are more likely to have fewer complications. Women over 35 are more inclined to have a longer labor period, which could potentially result in death of the mother or fetus. Women under 16 and over 35 have a higher risk of preterm labor (premature baby), and this risk increases for women in poverty, African Americans, and women who smoke. Young mothers are more likely to engage in high risk behaviors, such as using alcohol, drugs, or smoking, resulting in negative consequences for the fetus. Premature babies from young mothers are more likely to have neurological defects that will influence their coping capabilities - irritability, trouble sleeping, constant crying for example. There is a risk of Down syndrome for infants born to those aged over 40 years. Young teenaged mothers (younger than 16) and mothers over 35 are more exposed to the risks of miscarriages, premature births, and birth defects.
- Drug use
Eleven percent of fetuses are exposed to illicit drug use during pregnancy. Maternal drug use occurs when drugs ingested by the pregnant woman are metabolized in the placenta and then transmitted to the fetus. When using drugs (narcotics), there is a greater risk of birth defects, low birth weight, and a higher rate of death in infants or stillbirths. Drug use will influence extreme irritability, crying, and risk for SIDS once the fetus is born. The chemicals in drugs can cause an addiction in the babies once they are born. Marijuana will slow the fetal growth rate and can result in premature delivery. It can also lead to low birth weight, a shortened gestational period and complications in delivery. Heroin will cause interrupted fetal development, stillbirths, and can lead to numerous birth defects. Heroin can also result in premature delivery, creates a higher risk of miscarriages, result in facial abnormalities and head size, and create gastrointestinal abnormalities in the fetus. There is an increased risk for SIDS, dysfunction in the central nervous system, and neurological dysfunctions including tremors, sleep problems, and seizures. The fetus is also put at a great risk for low birth weight and respiratory problems. Cocaine use results in a smaller brain, which results in learning disabilities for the fetus. Cocaine puts the fetus at a higher risk of being stillborn or premature. Cocaine use also results in low birthweight, damage to the central nervous system, and motor dysfunction.
- Alcohol
Alcohol use leads to disruptions of the fetus's brain development, interferes with the fetus's cell development and organization, and affects the maturation of the central nervous system. Alcohol use can lead to heart and other major organ defects, such as small brain, which will affect the fetus's learning behaviors. Alcohol use during pregnancy can cause behavioral problems in a child, mental problems or retardation and facial abnormalities - meaning smaller eyes, thin upper lip, and little groove between the nose and lips. Use can also increase the risk of miscarriages and stillbirths, or low birth weight. Fetal alcohol syndrome (FAS) is a developmental disorder that is a consequence of too much alcohol intake by the mother during pregnancy. Children with FAS have a variety of distinctive facial features, brain abnormalities, and cognitive deficits.[5]
- Smoking and Nicotine
When a mother smokes during pregnancy the fetus is exposed to nicotine, tar, and carbon monoxide. Nicotine results in less blood flow to the fetus because it constricts the blood vessels. Carbon monoxide reduces the oxygen flow to the fetus. The reduction of blood and oxygen flow results in stillbirth, low birth weight, and ectopic pregnancy. There is an increase of risk of sudden death syndrome (SIDS) in infants. Nicotine also increases the risk for miscarriages and premature births or infant mortality. There has been a link from smoking during pregnancy that led to asthma in childhood. Low birth weight and premature births can also increase the risk of asthma if a mother smoked during pregnancy because of the effects on the respiratory system of the fetus.
- Diseases
If a mother is infected with a disease, the placenta cannot always filter out pathogens. Babies can be born with venereal diseases transmitted by the mother.
- Mother's diet and physical health
An adequate nutrition is needed for a healthy fetus. A lack of iron results in anemia in the fetus, the lack of calcium can result in poor bone and teeth formation, and the lack of protein can lead to a smaller fetus and mental retardation. Generally prenatal care gives an improved result in the newborn.[21]
- Mother's prenatal depression
A study found that mother's prenatal depression was associated with adverse perinatal outcomes such as slower fetal growth rates. It appears that prenatal maternal cortisol levels play a role in mediating these outcomes.[22]
- Environmental toxins
Exposure to environmental toxins in pregnancy lead to higher rates of miscarriage, sterility, and birth defects. Toxins include fetal exposure to lead, mercury, and ethanol or hazardous environments.
- Low birth weight
Low birth weight increases an infants risk of long-term growth and cognitive and language deficits. It also results in a shortened gestational period and can lead to prenatal complications.
Fetal hematology
Fetal hematopoiesis first takes place in the yolk sac. The function is transferred to liver by 10th week of gestation and to spleen and bone marrow beyond that. The total blood volume is about 125 ml/kg fetal body weight near term.
Erythrocytes
Fetus produces megaloblastic red blood cells early in development, which become normoblastic near term. Life span of fetal RBCs is 80 days. Rh antigen appears at about 40 days of gestation.
Leukocytes
Fetus starts producing leukocytes at 2 months gestation mainly from thymus and spleen. Lymphocytes derived from thymus are called T lymphocytes, whereas the ones derived from bone marrow are called B lymphocytes. Both these populations of lymphocytes have short-lived and long-lived groups. Short-lived T lymphocytes usually reside in thymus, bone marrow and spleen; whereas long-lived T lymphocytes reside in blood stream. Plasma cells are derived from B lymphocytes and their life in fetal blood is 0.5 to 2 days.
Fetal endocrinology
Thyroid gland is the first to develop in fetus at 4th week of gestation. Insulin secretion in fetus starts around 12th week of gestation.
References
- ↑ Definitions and Indicators in Family Planning. Maternal & Child Health and Reproductive Health. By European Regional Office, World Health Organization. Revised March 1999 & January 2001. In turn citing: WHO Geneva, WHA20.19, WHA43.27, Article 23
- ↑ Singh, Meharban (2010). Care of the Newborn. p. 7. Edition 7. ISBN 9788170820536
- ↑ patient.info » PatientPlus » Antepartum Haemorrhage Last Updated: 5 May 2009
- ↑ The Royal Women’s Hospital > antepartum haemorrhage Retrieved on Jan 13, 2009
- 1 2 Schacter, Daniel (2009). "11-Development". Psychology Second Edition. United States of America: Worth Publishers. ISBN 978-1-4292-3719-2.
- ↑ Wilcox AJ, Baird DD, Weinberg CR (1999). "Time of implantation of the conceptus and loss of pregnancy". N. Engl. J. Med. 340 (23): 1796–9. doi:10.1056/NEJM199906103402304. PMID 10362823.
- ↑ Moore L. Keith. (2008). Before We Are Born: Essentials of Embryology and Birth Defects. Philadelphia, PA: Saunders/Elsevier. ISBN 978-1-4160-3705-7.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 William J. Larsen (2001). Human embryology. Edinburgh: Churchill Livingstone. ISBN 0-443-06583-7.
- ↑ Scott F. Gilbert; with a chapter on plant development by Susan R. Singer (2000). Developmental biology. Sunderland, Mass: Sinauer Associates. ISBN 0-87893-243-7.
- ↑ 3D Pregnancy (large image of fetus at 4 weeks after fertilization). Retrieved 2007-08-28. A rotatable 3D version of this photo is available here, and a sketch is available here.
- ↑ "7 Weeks Pregnant – Symptoms, Fetal Development, Tips". Retrieved 2016-07-18.
- ↑ Wagner F, Erdösová B, Kylarová D (December 2004). "Degradation phase of apoptosis during the early stages of human metanephros development". Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 148 (2): 255–6. doi:10.5507/bp.2004.054. PMID 15744391.
- ↑ 3D Pregnancy (large image of fetus at 10 weeks after fertilization). Retrieved 2007-08-28. A rotatable 3D version of this photo is available here, and a sketch is available here.
- ↑ Jeve, Y (13 October 2011). "Accuracy of first-trimester ultrasound in the diagnosis of early embryonic demise: a systematic review". Ultrasound in Obstetrics and Gynecology. 38 (5): 489–496. doi:10.1002/uog.10108. Retrieved 26 October 2015.
- ↑ Mazza V, Falcinelli C, Paganelli S, et al. (June 2001). "Sonographic early fetal gender assignment: a longitudinal study in pregnancies after in vitro fertilization". Ultrasound Obstet Gynecol. 17 (6): 513–6. doi:10.1046/j.1469-0705.2001.00421.x. PMID 11422974.
- ↑ 3D Pregnancy (large image of fetus at 18 weeks after fertilization). Retrieved 2007-08-28. A rotatable 3D version of this photo is available here, and a sketch is available here.
- ↑ 3D Pregnancy (large image of fetus at 38 weeks after fertilization). Retrieved 2007-08-28. A rotatable 3D version of this photo is available here, and a sketch is available here.
- 1 2 Daftary, Shirish; Chakravarti, Sudip (2011). Manual of Obstetrics, 3rd Edition. Elsevier. pp. 1-16. ISBN 9788131225561.
- ↑ , "Prenatal Factors in Schizophrenia", 2012-07-10
- ↑ Lawn JE, Cousens S, Zupan J (2005). "4 million neonatal deaths: when? Where? Why?". The Lancet. 365: 891–900. doi:10.1016/s0140-6736(05)71048-5.
- ↑ "What is prenatal care and why is it important?". www.nichd.nih.gov.
- ↑ Diego M. A.; Field T.; Hernandez-Reif M.; Schanberg S.; Kuhn C.; Gonzalez-Quintero V. H. (2009). "Prenatal depression restricts fetal growth". Early human development. 85 (1): 65–70. doi:10.1016/j.earlhumdev.2008.07.002. PMC 2651570
 . PMID 18723301.
. PMID 18723301.
Further reading
- MedlinePlus Encyclopedia Fetal development
- Moore, Keith L. The Developing Human (3rd ed.). Philadelphia PA: W.B. Saunders Company.
- Wilcox AJ, Baird DD, Weinberg CR (June 1999). "Time of implantation of the conceptus and loss of pregnancy". N. Engl. J. Med. 340 (23): 1796–9. doi:10.1056/NEJM199906103402304. PMID 10362823.
- Ljunger E, Cnattingius S, Lundin C, Annerén G (November 2005). "Chromosomal anomalies in first-trimester miscarriages". Acta Obstet Gynecol Scand. 84 (11): 1103–7. doi:10.1111/j.0001-6349.2005.00882.x. PMID 16232180.
- Newman, Barbara; Newman, Philip (10 March 2008). "The Period of Pregnancy and Prenatal Development". Development Through Life: A Psychosocial Approach. Cengage Learning. ISBN 0-495-55341-7.
- "Prenatal Development - Prenatal Environmental Influences - Mother, Birth, Fetus, and Pregnancy." Social Issues Reference. Version Child Development Vol. 6. N.p., n.d. Web. 19 Nov. 2012. <http://social.jrank.org/pages/515/Prenatal-Development-Prenatal-Environmental-Influences.html>.
- Niedziocha, Laura. "The Effects Of Drugs And Alcohol On Fetal Development | LIVESTRONG.COM." LIVESTRONG.COM - Lose Weight & Get Fit with Diet, Nutrition & Fitness Tools | LIVESTRONG.COM. N.p., 4 Sept. 2011. Web. 19 Nov. 2012. <http://www.livestrong.com/article/535499-the-effects-of-drugs-and-alcohol-on-fetal-development/>.
- Jaakkola, JJ; Gissler, M (January 2004). "Maternal smoking in pregnancy, fetal development, and childhood asthma.". American Journal of Public Health. 94 (1): 136–40. doi:10.2105/ajph.94.1.136. PMC 1449839
 . PMID 14713711.
. PMID 14713711. - Gutbrod, T (1 May 2000). "Effects of gestation and birth weight on the growth and development of very low birthweight small for gestational age infants: a matched group comparison". Archives of Disease in Childhood: Fetal and Neonatal Edition. 82 (3): 208F–214. doi:10.1136/fn.82.3.F208. PMC 1721075
 . PMID 10794788.
. PMID 10794788. - Brady, Joanne P., Marc Posner, and Cynthia Lang. "Risk and Reality: The Implications of Prenatal Exposure to Alcohol and Other Drugs ." ASPE. N.p., n.d. Web. 19 Nov. 2012. <http://aspe.hhs.gov/hsp/cyp/drugkids.htm>.
- Pregnancy Approach (eBook ed.). Lauren Lee. 2013.
External links
| Wikimedia Commons has media related to Embryology. |
- Chart of human fetal development, U.S. National Library of Medicine (NLM)
- U.K. Human Fertilisation and Embryology Authority (HFEA), regulatory agency overseeing the use of gametes and embryos in fertility treatment and research