Polymyxin B
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | Topical, Intramuscular, Intravenous, Intrathecal, or Ophthalmic |
| ATC code | A07AA05 (WHO) J01XB02 (WHO) S01AA18 (WHO) S02AA11 (WHO) S03AA03 (WHO) QJ51XB02 (WHO) |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
1405-20-5 |
| PubChem (CID) | 5702105 |
| DrugBank |
DB00781 |
| ChEMBL |
CHEMBL1201283 |
| NIAID ChemDB | 007797 |
| ECHA InfoCard | 100.014.340 |
| Chemical and physical data | |
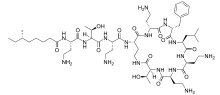
| Formula | C56H100N16O17S |
| Molar mass | 1301.56 g/mol |
| | |
Polymyxin B is an antibiotic primarily used for resistant Gram-negative infections. It is derived from the bacterium Bacillus polymyxa. Polymyxin B is composed of a number of related compounds (see "Mixture composition"). It has a bactericidal action against almost all Gram-negative bacilli except the Proteus and Neisseria genera. Polymyxins bind to the cell membrane and alter its structure, making it more permeable. The resulting water uptake leads to cell death. Polymyxins are cationic, basic peptides that act like detergents (surfactants). Side effects include neurotoxicity and acute renal tubular necrosis. Polymyxins are used in the topical first-aid preparation Neosporin.
- Family of polypeptides with attached fatty acid; cationic detergent at physiological pH, both hydrophilic and hydrophobic properties
- Bactericidal for gram-negative; little to no effect on gram-positive, since cell wall is too thick to permit access to membrane
Mechanism of action
- Alters bacterial outer membrane permeability by binding to a negatively charged site in the lipopolysaccharide layer, which has an electrostatic attraction for the positively charged amino groups in the cyclic peptide portion (this site normally is a binding site for calcium and magnesium counter ions); the result is a destabilized outer membrane
- Fatty acid portion dissolves in hydrophobic region of cytoplasmic membrane and disrupts membrane integrity
- Leakage of cellular molecules, inhibition of cellular respiration
- Binds and inactivates endotoxin[1]
- Relative absence of selective toxicity: nonspecific for cell membranes of any type, highly toxic.
Mixture composition
Polymyxin B is composed of polymyxins B1, B1-I, B2, B3, and B6. Polymyxins B1 and B2 are considered major components. These related components are structurally identical with the exception of a variable fatty acid group on each fraction. Results from in vitro studies have shown marginal differences in MIC data when comparing the fractions.[2]
Research application
In addition to its antibiotic function, polymyxin B has been used to clear endotoxin contamination in reagents. Polymyxin B is also used to induce envelope stress in order to study the organisms response to such stress. Polymyxin envelope stress assays such as this have been used for the study of sRNA responses in Salmonella enterica.[3]
Endotoxin adsorption cartridge
An endotoxin removal cartridge (Toraymyxin) is a blood purification medical device and it uses polymyxin B as immobilized adsorbent.[4] Toray Industries developed the treatment.
Spectrum of susceptibility
Polymyxin B has been used to treat urinary tract infections and meningitis caused by Pseudomonas aeruginosa and Haemophilus influenzae, respectively. The following represents MIC susceptibility data for a few medically significant microorganisms.
- Haemophilus influenzae: ≥0.8 μg/ml
- Pseudomonas aeruginosa: 0.25 μg/ml - 1 μg/ml
References
- ↑ Cardoso LS, Araujo MI, Góes AM, Pacífico LG, Oliveira RR, Oliveira SC (2007). "Polymyxin B as inhibitor of LPS contamination of Schistosoma mansoni recombinant proteins in human cytokine analysis". Microb. Cell Fact. 6: 1. doi:10.1186/1475-2859-6-1. PMC 1766364
 . PMID 17201926.
. PMID 17201926. - ↑ Orwa, J. A., et al "Isolation and Structural Characterization of Polymyxin B Components." Isolation and Structural Characterization of Polymyxin B Components 912.2 (2001): 369-73. Sciencedirect. Web. 15 Jan. 2013.
- ↑ Hinton, Jay; Magali Hébrard; Carsten Kröger; Shabarinath Srikumar; Aoife Colgan; Kristian Händler (April 2012). "sRNAs and the virulence of Salmonella enterica serovar Typhimurium". RNA Biology. 9 (4): 437–445. doi:10.4161/rna.20480. PMC 3384567
 . PMID 22546935.
. PMID 22546935. - ↑ Shoji H. (February 2003). "Extracorporeal endotoxin removal for the treatment of sepsis: endotoxin adsorption cartridge (Toraymyxin).". Therapeutic Apheresis and Dialysis. 7 (1): 108–114. doi:10.1046/j.1526-0968.2003.00005.x. PMID 12921125.
- ↑ http://www.toku-e.com/Assets/MIC/Polymyxin%20B%20sulfate.pdf