Rheumatoid arthritis
| Rheumatoid arthritis | |
|---|---|
|
A hand affected by rheumatoid arthritis | |
| Classification and external resources | |
| Specialty | Rheumatology |
| ICD-10 | M05-M06 |
| ICD-9-CM | 714 |
| OMIM | 180300 |
| DiseasesDB | 11506 |
| MedlinePlus | 000431 |
| eMedicine | article/331715 article/1266195 article/305417 article/401271 article/335186 article/808419 |
| Patient UK | Rheumatoid arthritis |
| MeSH | D001172 |
Rheumatoid arthritis (RA) is a long-lasting autoimmune disorder that primarily affects joints. It typically results in warm, swollen, and painful joints. Pain and stiffness often worsen following rest. Most commonly, the wrist and hands are involved, with the same joints typically involved on both sides of the body. The disease may also affect other parts of the body. This may result in a low red blood cell count, inflammation around the lungs, and inflammation around the heart. Fever and low energy may also be present.[1] Often, symptoms come on gradually over weeks to months.[2]
While the cause of rheumatoid arthritis is not clear, it is believed to involve a combination of genetic and environmental factors. The underlying mechanism involves the body's immune system attacking the joints. This results in inflammation and thickening of the joint capsule. It also affects the underlying bone and cartilage.[1] The diagnosis is made mostly on the basis of a person's signs and symptoms.[2] X-rays and laboratory testing may support a diagnosis or exclude other diseases with similar symptoms.[1] Other diseases that may present similarly include systemic lupus erythematosus, psoriatic arthritis, and fibromyalgia among others.[2]
The goal of treatment is to reduce pain, decrease inflammation, and improve a person's overall functioning. This may be helped by balancing rest and exercise, the use of splints and braces, or the use of assistive devices. Pain medications, steroids, and NSAIDs are frequently used to help with symptoms. A group of medications called disease-modifying antirheumatic drugs (DMARDs) may be used to try to slow the progression of disease. They include the medications hydroxychloroquine and methotrexate.[1] Biological DMARDs may be used when disease does not respond to other treatments.[3] However, they may have a greater rate of adverse effects.[4] Surgery to repair, replace, or fuse joints may help in certain situations.[1] Most alternative medicine treatments are not supported by evidence.[5][6]
RA affects between 0.5 and 1% of adults in the developed world with between 5 and 50 per 100,000 people newly developing the condition each year.[3] Onset is most frequent during middle age and women are affected 2.5 times as frequently as men.[1] In 2013, it resulted in 38,000 deaths up from 28,000 deaths in 1990.[7] The first recognized description of RA was made in 1800 by Dr. Augustin Jacob Landré-Beauvais (1772–1840) of Paris.[8] The term rheumatoid arthritis is based on the Greek for watery and inflamed joints.[9]
Signs and symptoms
RA primarily affects joints, but it also affects other organs in more than 15–25% of individuals.[10]
Joints
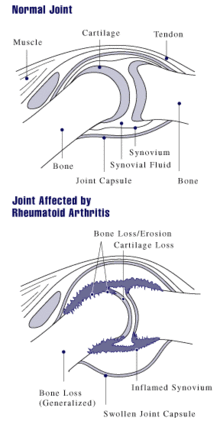
Arthritis of joints involves inflammation of the synovial membrane. Joints become swollen, tender and warm, and stiffness limits their movement. With time, multiple joints are affected (polyarthritis). Most commonly involved are the small joints of the hands, feet and cervical spine, but larger joints like the shoulder and knee can also be involved.[11]:1089 Synovitis can lead to tethering of tissue with loss of movement and erosion of the joint surface causing deformity and loss of function.[2]
RA typically manifests with signs of inflammation, with the affected joints being swollen, warm, painful and stiff, particularly early in the morning on waking or following prolonged inactivity. Increased stiffness early in the morning is often a prominent feature of the disease and typically lasts for more than an hour. Gentle movements may relieve symptoms in early stages of the disease. These signs help distinguish rheumatoid from non-inflammatory problems of the joints, often referred to as osteoarthritis. In arthritis of non-inflammatory causes, signs of inflammation and early morning stiffness are less prominent with stiffness typically less than one hour, and movements induce pain caused by mechanical arthritis.[12] The pain associated with RA is induced at the site of inflammation and classified as nociceptive as opposed to neuropathic.[13] The joints are often affected in a fairly symmetrical fashion, although this is not specific, and the initial presentation may be asymmetrical.[11]:1089
As the pathology progresses the inflammatory activity leads to tendon tethering and erosion and destruction of the joint surface, which impairs range of movement and leads to deformity. The fingers may suffer from almost any deformity depending on which joints are most involved. Specific deformities, which also occur in osteoarthritis, include ulnar deviation, boutonniere deformity (also "buttonhole deformity", flexion of proximal interphalangeal joint and extension of distal interphalangeal joint), swan neck deformity (hyperextension at proximal interphalangeal joint and flexion at distal interphalangeal joint) and "Z-thumb." "Z-thumb" or "Z-deformity" consists of hyperextension of the interphalangeal joint, fixed flexion and subluxation of the metacarpophalangeal joint and gives a "Z" appearance to the thumb.[11]:1089 The hammer toe deformity may be seen. In the worst case, joints are known as arthritis mutilans due to the mutilating nature of the deformities.[14] "Spindling of fingers" of hand occurs due to swelling of the PIP but not DIP joints. "Piano key movement" of the ulnar styloid occurs due to inflammation around the ulnar styloid and tenosynovitis of extensor carpi ulnaris.
Skin
The rheumatoid nodule, which is sometimes in the skin, is the most common non joint feature.[15] They occur in 30% of people.[15] It is a type of inflammatory reaction known to pathologists as a "necrotizing granuloma". The initial pathologic process in nodule formation is unknown but may be essentially the same as the synovitis, since similar structural features occur in both. The nodule has a central area of fibrinoid necrosis that may be fissured and which corresponds to the fibrin-rich necrotic material found in and around an affected synovial space. Surrounding the necrosis is a layer of palisading macrophages and fibroblasts, corresponding to the intimal layer in synovium and a cuff of connective tissue containing clusters of lymphocytes and plasma cells, corresponding to the subintimal zone in synovitis. The typical rheumatoid nodule may be a few millimetres to a few centimetres in diameter and is usually found over bony prominences, such as the elbow, the heel, the knuckles, or other areas that sustain repeated mechanical stress. Nodules are associated with a positive RF (rheumatoid factor) titer and severe erosive arthritis. Rarely, these can occur in internal organs or at diverse sites on the body..
Several forms of vasculitis occur in RA. A benign form occurs as microinfarcts around the nailfolds. More severe forms include livedo reticularis, which is a network (reticulum) of erythematous to purplish discoloration of the skin caused by the presence of an obliterative cutaneous capillaropathy..
Other, rather rare, skin associated symptoms include pyoderma gangrenosum, Sweet's syndrome, drug reactions, erythema nodosum, lobe panniculitis, atrophy of finger skin, palmar erythema, diffuse thinning (rice paper skin), and skin fragility (often worsened by corticosteroid use)..
Lungs
Fibrosis of the lungs is a recognized response to rheumatoid disease. It is also a rare but well-recognized consequence of therapy (for example with methotrexate and leflunomide). Caplan's syndrome describes lung nodules in individuals with RA and additional exposure to coal dust. Pleural effusions are also associated with RA. Another complication of RA is Rheumatoid Lung Disease. It is estimated that about one-quarter of Americans with RA develop Rheumatoid Lung Disease.[16]
Kidneys
Renal amyloidosis can occur as a consequence of chronic inflammation.[17] RA may affect the kidney glomerulus directly through a vasculopathy or a mesangial infiltrate but this is less well documented (though this is not surprising, considering immune complex-mediated hypersensitivities are known for pathogenic deposition of immune complexes in organs where blood is filtered at high pressure to form other fluids, such as urine and synovial fluid[18]). Treatment with penicillamine and gold salts are recognized causes of membranous nephropathy.
Heart and blood vessels
People with RA are more prone to atherosclerosis, and risk of myocardial infarction (heart attack) and stroke is markedly increased.[19][20] Other possible complications that may arise include: pericarditis, endocarditis, left ventricular failure, valvulitis and fibrosis.[21] Many people with RA do not experience the same chest pain that others feel when they have angina or myocardial infarction. To reduce cardiovascular risk, it is crucial to maintain optimal control of the inflammation caused by RA (which may be involved in causing the cardiovascular risk), and to use exercise and medications appropriately to reduce other cardiovascular risk factors such as blood lipids and blood pressure. Doctors who treat people with RA should be sensitive to cardiovascular risk when prescribing anti-inflammatory medications, and may want to consider prescribing routine use of low doses of aspirin if the gastrointestinal effects are tolerable.[21]
Other
- Eyes
- The eye can be directly affected in the form of episcleritis or scleritis. Scleritis when severe can very rarely progress to perforating scleromalacia. Rather more common is the indirect effect of keratoconjunctivitis sicca, which is a dryness of eyes and mouth caused by lymphocyte infiltration of lacrimal and salivary glands. When severe, dryness of the cornea can lead to keratitis and loss of vision. Preventive treatment of severe dryness with measures such as nasolacrimal duct blockage is important.
- Liver
- Liver problems in people with rheumatoid arthritis may be due to the underlying disease process or as a result of the medications used to treat the disease.[22] A coexisting autoimmune liver disease, such as primary biliary cirrhosis or autoimmune hepatitis may also cause problems.[22]
- Blood
- Anemia is by far the most common abnormality of the blood cells which can be caused by a variety of mechanisms. The chronic inflammation caused by RA leads to raised hepcidin levels, leading to anemia of chronic disease where iron is poorly absorbed and also sequestered into macrophages. RA may also cause a warm autoimmune hemolytic anemia.[23] The red cells are of normal size and color (normocytic and normochromic). A low white blood cell count usually only occurs in people with Felty's syndrome with an enlarged liver and spleen. The mechanism of neutropenia is complex. An increased platelet count occurs when inflammation is uncontrolled.
- Neurological
- Peripheral neuropathy and mononeuritis multiplex may occur. The most common problem is carpal tunnel syndrome caused by compression of the median nerve by swelling around the wrist. Atlanto-axial subluxation can occur, owing to erosion of the odontoid process and/or transverse ligaments in the cervical spine's connection to the skull. Such an erosion (>3mm) can give rise to vertebrae slipping over one another and compressing the spinal cord. Clumsiness is initially experienced, but without due care, this can progress to quadriplegia.
- Constitutional symptoms
- Constitutional symptoms including fatigue, low grade fever, malaise, morning stiffness, loss of appetite and loss of weight are common systemic manifestations seen in people with active RA.
- Bones
- Local osteoporosis occurs in RA around inflamed joints. It is postulated to be partially caused by inflammatory cytokines. More general osteoporosis is probably contributed to by immobility, systemic cytokine effects, local cytokine release in bone marrow and corticosteroid therapy.
Causes
RA is a chronic autoimmune disorder the causes of which are not completely understood. It is a systemic (whole body) disorder principally affecting synovial tissues. There is no evidence that physical and emotional effects or stress could be a trigger for the disease. The many negative findings suggest that either the trigger varies, or that it might, in fact, be a chance event inherent with the immune response.[26]
Half of the risk for RA is believed to be genetic.[3] It is strongly associated with the inherited tissue type major histocompatibility complex (MHC) antigen HLA-DRB1 (most specifically the shared epitope alleles, including *0401 and born 0404), and the genes PTPN22 and PADI4—hence family history is an important risk factor.[27][28] Inheriting the PTPN22 gene has been shown to double a person's susceptibility to RA. PADI4 has been identified as a major risk factor in people of Asian descent, but not in those of European descent.[29] First-degree relatives prevalence rate is 2–3% and disease genetic concordance in monozygotic twins is approximately 15–20%.[30][31]
Smoking is the most significant non-genetic risk[3] with RA being up to three times more common in smokers than non-smokers, particularly in men, heavy smokers, and those who are rheumatoid factor positive.[32] Modest alcohol consumption may be protective.[33]
Epidemiological studies have confirmed a potential association between RA and two herpesvirus infections: Epstein-Barr virus (EBV) and Human Herpes Virus 6 (HHV-6).[34] Individuals with RA are more likely to exhibit an abnormal immune response to EBV and have high levels of anti-EBV antibodies.[35]
Vitamin D deficiency is more common in people with rheumatoid arthritis than in the general population.[36][37] However, whether vitamin D deficiency is a cause or a consequence of the disease remains unclear.[38] 1α,25-dihydroxyvitamin D3 (1,25D), an active metabolite of vitamin D, affects bone metabolism indirectly through control of calcium and phosphate homeostasis. Interaction between 1,25D and the vitamin D receptor (VDR) affects the production of RANKL and delays osteoclastogenesis.[39] Some trials have found a decreased risk for RA with vitamin D supplementation while others have not.[37]
Pathophysiology
Both genetic, as well as environmental factors, are implicated in the pathophysiology of the disease. Smoking is the main environmental risk to RA.[3] 50% of the risk of having RA is attributable to genetic factors.[3] No infectious agent has been consistently linked with RA and there is no evidence of disease clustering to indicate its infectious etiology.[40] HLA-DR4 is the major genetic factor implicated – but its relative importance varies across ethnic groups.[40][41] Related allotypes of MHC Class II and the T cell-associated protein PTPN22 has also been found associated in many studies.[41]
RA primarily starts as a state of persistent cellular activation leading to autoimmunity and immune complexes in both joints and other, organs where it manifests. The initial site of disease is the synovial membrane, where swelling and congestion leads to infiltration by immune cells. The various phases of progression of RA are:[14]
- Initiation phase, due to non-specific inflammation.
- Amplification phase, due to T cell activation
- Chronic inflammatory phase with tissue injury, due to cytokines IL–1, TNF-alpha and IL–6.
The factors that allow an abnormal immune response, once initiated, to become permanent and chronic, are becoming more clearly understood. The genetic association with HLA-DR4, as well as the newly discovered associations with the gene PTPN22 and with two additional genes,[41] all implicate altered thresholds in regulation of the adaptive immune response. It has also become clear from recent studies that these genetic factors may interact with the most clearly defined environmental risk factor for RA, namely cigarette smoking.[32][42] Other environmental factors also appear to modulate the risk of acquiring RA, and hormonal factors in the individual may explain some features of the disease, such as the higher occurrence in women, the not-infrequent onset after child-birth, and the (slight) modulation of disease risk by hormonal medications. Exactly how altered regulatory thresholds allow the triggering of a specific autoimmune response remains uncertain. However, one possibility is that negative feedback mechanisms that normally maintain tolerance of self are overtaken by aberrant positive feedback mechanisms for certain antigens such as IgG Fc (bound by RF) and citrullinated fibrinogen (bound by ACPA) (see the entry on autoimmunity).The debate on the relative roles of immune complexes and T cell products in inflammation in RA has continued for 30 years. There is little doubt that both B and T cells are essential to the disease. However, there is good evidence for neither cell being necessary at the site of inflammation. This tends to favor immune complexes (based on antibody synthesized elsewhere) as the initiators, even if not the sole perpetuators of inflammation. The presence of autoantibodies to IgGFc, known as rheumatoid factors (RF), and antibodies to citrullinated peptides (ACPA) is an integral part of RA disease process. As is the case with many other autoimmune diseases, people with RA have abnormally glycosylated antibodies.[43] It is believed that these glycan (oligosaccharide) alterations promote joint inflammation.[43]
Once the abnormal immune response has become established (which may take several years before any symptoms occur), plasma cells derived from B lymphocytes produce rheumatoid factors and ACPA of the IgG and IgM classes in large quantities. These are not deposited in the way that they are in systemic lupus. Rather, they activate macrophages through Fc receptor and complement binding, which seems to play an important role in the intense inflammatory response present in RA.[44] Binding of an autoreactive antibody to the Fc receptors is mediated through the antibody's N-glycans, which are altered to promote inflammation in people with RA.[43] This contributes to inflammation of the synovium, in terms of edema, vasodilation and infiltration by activated T-cells (mainly CD4 in nodular aggregates and CD8 in diffuse infiltrates). Synovial macrophages and dendritic cells further function as antigen presenting cells by expressing MHC class II molecules, leading to an established local immune reaction in the tissue. The disease progresses in concert with the formation of granulation tissue at the edges of the synovial lining (pannus) with extensive angiogenesis and production of enzymes that cause tissue damage. Modern pharmacological treatments of RA target these mediators. Once the inflammatory reaction is established, the synovium thickens, the cartilage and the underlying bone begins to disintegrate and evidence of joint destruction accrues.
TNF (alpha) plays a major role in the pathogenesis of RA. There are several theories on how TNF release happens in disease process. If TNF release is stimulated by B cell products in the form of RF or ACPA -containing immune complexes, through activation of immunoglobulin Fc receptors, then RA can be seen as a form of Type III hypersensitivity.[45][46] If TNF release is stimulated by T cell products such as interleukin-17 it might be considered closer to type IV hypersensitivity although this terminology may be getting somewhat dated and unhelpful.[47]
Although TNF appears to be the dominant, other cytokines (chemical mediators) are likely to be involved in inflammation in RA. Blockade of TNF does not benefit all persons or all tissues (lung disease and nodules may get worse). Blockade of IL-1, IL-15 and IL-6 also have beneficial effects and IL-17 may be important. Constitutional symptoms such as fever, malaise, loss of appetite and weight loss are also caused by cytokines released into the blood stream. As with most autoimmune diseases, it is important to distinguish between the cause(s) that trigger the process and those that may permit it to persist and progress.
Diagnosis
Imaging
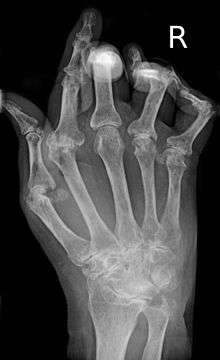
X-rays of the hands and feet are generally performed in people with many joints affected. In RA, there may be no changes in the early stages of the disease or the x-ray may demonstrate juxta-articular osteopenia, soft tissue swelling, and loss of joint space. As the disease advances, there may be bony erosions and subluxation. X-rays of other joints may be taken if symptoms of pain or swelling occur in those joints.
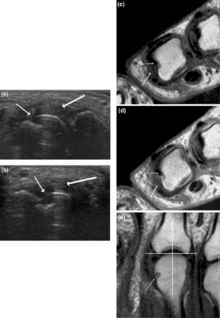
Other medical imaging techniques such as magnetic resonance imaging (MRI) and ultrasound are also used in RA.[14]
There have been technical advances in ultrasonography. High-frequency transducers (10 MHz or higher) have improved the spatial resolution of ultrasound images; these images can depict 20% more erosions than conventional radiography. Also, color Doppler and power Doppler ultrasound, which show vascular signals of active synovitis depending on the degree of inflammation, are useful in assessing synovial inflammation. This is important, since in the early stages of RA, the synovium is primarily affected, and synovitis seems to be the best predictive marker of future joint damage.[48]
Blood tests
When RA is clinically suspected, testing for the presence of rheumatoid factor (RF, a non-specific antibody) and (ACPAs) may be required.[49] A negative RF does not rule out RA; rather, the arthritis is called seronegative. This is the case in about 15% of people with RA.[50][51] During the first year of illness, rheumatoid factor is more likely to be negative with some individuals converting to seropositive status over time. RF is also seen in other illnesses, for example Sjögren's syndrome, hepatitis C, systemic lupus erythematosus, chronic infections and in approximately 10% of the healthy population, therefore the test is not very specific.[14]
Because of this low specificity, new serological tests have been developed, which test for the presence of the anti-citrullinated protein antibodies (ACPAs) or anti-CCP. Like RF, these tests are positive in only a proportion (67%) of all RA cases, but are rarely positive if RA is not present, giving it a specificity of around 95%.[50] As with RF, there is evidence for ACPAs being present in many cases even before onset of clinical disease.[14]
The most common tests for ACPAs are the anti-CCP (cyclic citrullinated peptide) test and the Anti-MCV assay (antibodies against mutated citrullinated Vimentin). Recently a serological point-of-care test (POCT) for the early detection of RA has been developed. This assay combines the detection of rheumatoid factor and anti-MCV for diagnosis of RA and shows a sensitivity of 72% and specificity of 99.7%.[52][53]
Also, several other blood tests are usually done to allow for other causes of arthritis, such as lupus erythematosus. The erythrocyte sedimentation rate (ESR), C-reactive protein, full blood count, kidney function, liver enzymes and other immunological tests (e.g., antinuclear antibody/ANA) are all performed at this stage. Elevated ferritin levels can reveal hemochromatosis, a mimic of RA, or be a sign of Still's disease, a seronegative, usually juvenile, variant of rheumatoid arthritis.
Classification Criteria
In 2010 the 2010 ACR / EULAR Rheumatoid Arthritis Classification Criteria were introduced.[54][55] The new criterion is not a diagnostic criterion but a classification criterion to identify disease with a high likelihood of developing a chronic form.[14] However a score of 6 or greater unequivocally classifies a person with a diagnosis of rheumatoid arthritis.
These new classification criteria overruled the "old" ACR criteria of 1987 and are adapted for early RA diagnosis. The "new" classification criteria, jointly published by the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) establish a point value between 0 and 10. Four areas are covered in the diagnosis:[54]
- joint involvement, designating the metacarpophalangeal joints, proximal interphalangeal joints, the interphalangeal joint of the thumb, second through fifth metatarsophalangeal joint and wrist as small joints, and shoulders, elbows, hip joints, knees, and ankles as large joints:
- Involvement of 1 large joint gives 0 points
- Involvement of 2–10 large joints gives 1 point
- Involvement of 1–3 small joints (with or without involvement of large joints) gives 2 points
- Involvement of 4–10 small joints (with or without involvement of large joints) gives 3 points
- Involvement of more than 10 joints (with involvement of at least 1 small joint) gives 5 points
- serological parameters – including the rheumatoid factor as well as ACPA – "ACPA" stands for "anti-citrullinated protein antibody":
- Negative RF and negative ACPA gives 0 points
- Low-positive RF or low-positive ACPA gives 2 points
- High-positive RF or high-positive ACPA gives 3 points
- acute phase reactants: 1 point for elevated erythrocyte sedimentation rate, ESR, or elevated CRP value (c-reactive protein)
- duration of arthritis: 1 point for symptoms lasting six weeks or longer
The new criteria accommodate to the growing understanding of RA and the improvements in diagnosing RA and disease treatment. In the "new" criteria serology and autoimmune diagnostics carries major weight, as ACPA detection is appropriate to diagnose the disease in an early state, before joints destructions occur. Destruction of the joints viewed in radiological images was a significant point of the ACR criteria from 1987.[56] This criterion no longer is regarded to be relevant, as this is just the type of damage that treatment is meant to avoid.
In clinical practice, the following criteria apply:
- two or more swollen joints
- morning stiffness lasting more than one hour for at least six weeks
- the detection of rheumatoid factors or autoantibodies against ACPA such as autoantibodies to mutated citrullinated vimentin can confirm the suspicion of RA. A negative autoantibody result does not exclude a diagnosis of RA.
Differential diagnoses
Several other medical conditions can resemble RA, and usually need to be distinguished from it at the time of diagnosis:[57][58]
- Crystal induced arthritis (gout, and pseudogout) – usually involves particular joints (knee, MTP1, heels) and can be distinguished with an aspiration of joint fluid if in doubt. Redness, asymmetric distribution of affected joints, pain occurs at night and the starting pain is less than an hour with gout.
- Osteoarthritis – distinguished with X-rays of the affected joints and blood tests, age (mostly older persons), starting pain less than an hour, a-symmetric distribution of affected joints and pain worsens when using joint for longer periods.
- Systemic lupus erythematosus (SLE) – distinguished by specific clinical symptoms and blood tests (antibodies against double-stranded DNA)
- One of the several types of psoriatic arthritis resembles RA – nail changes and skin symptoms distinguish between them
- Lyme disease causes erosive arthritis and may closely resemble RA – it may be distinguished by blood test in endemic areas
- Reactive arthritis (previously Reiter's disease) – asymmetrically involves heel, sacroiliac joints and large joints of the leg. It is usually associated with urethritis, conjunctivitis, iritis, painless buccal ulcers, and keratoderma blennorrhagica.
- Ankylosing spondylitis – this involves the spine, although an RA-like symmetrical small-joint polyarthritis may occur in the context of this condition.
- Hepatitis C – RA-like symmetrical small-joint polyarthritis may occur in the context of this condition. Hepatitis C may also induce Rheumatoid Factor auto-antibodies
Rarer causes that usually behave differently but may cause joint pains:[57]
- Sarcoidosis, amyloidosis, and Whipple's disease can also resemble RA.
- Hemochromatosis may cause hand joint arthritis.
- Acute rheumatic fever can be differentiated from RA by a migratory pattern of joint involvement and evidence of antecedent streptococcal infection. Bacterial arthritis (such as by Streptococcus) is usually asymmetric, while RA usually involves both sides of the body symmetrically.
- Gonococcal arthritis (another bacterial arthritis) is also initially migratory and can involve tendons around the wrists and ankles.
Monitoring progression
There are many tools available for monitoring remission in rheumatoid arthritis.
- DAS28
- Disease Activity Score of 28 joints (DAS28) is widely used as an indicator of RA disease activity and response to treatment, but is not always a reliable indicator of treatment effect.[59] The joints included in DAS28 are (bilaterally): proximal interphalangeal joints (10 joints), metacarpophalangeal joints (10), wrists (2), elbows (2), shoulders (2) and knees (2). When looking at these joints, both the number of joints with tenderness upon touching (TEN28) and swelling (SW28) are counted. In addition, the erythrocyte sedimentation rate (ESR) is measured. Also, the affected person makes a subjective assessment (SA) of disease activity during the preceding 7 days on a scale between 0 and 100, where 0 is "no activity" and 100 is "highest activity possible". With these parameters, DAS28 is calculated as:[60]
From this, the disease activity of the affected person can be classified as follows:[60]
| Current DAS28 | DAS28 decrease from initial value | |||
|---|---|---|---|---|
| > 1.2 | > 0.6 but ≤ 1.2 | ≤ 0.6 | ||
| ≤ 3.2 | Inactive | Good improvement | Moderate improvement | No improvement |
| > 3.2 but ≤ 5.1 | Moderate | Moderate improvement | Moderate improvement | No improvement |
| > 5.1 | Very active | Moderate improvement | No improvement | No improvement |
One major limitation of use of the DAS28 score in clinical setting is low-grade synovitis may be missed.
- Other
- Other tools to monitor remission in rheumatoid arthritis are: ACR-EULAR Provisional Definition of Remission of Rheumatoid arthritis, Simplified Disease Activity Index (SDAI) and Clinical Disease Activity Index (CDAI).[61]
Prevention
There is no known prevention for the condition other than the reduction of risk factors.[62]
Management
There is no cure for RA, but treatments can improve symptoms and slow the progress of the disease. Disease-modifying treatment has the best results when it is started early and aggressively.[63]
The goals of treatment are to minimize symptoms such as pain and swelling, to prevent bone deformity (for example, bone erosions visible in X-rays), and to maintain day-to-day functioning.[64] This can often be achieved using two main classes of medications: analgesics such as NSAIDs, and disease-modifying antirheumatic drugs (DMARDs).[65] RA should generally be treated with at least one specific anti-rheumatic medication.[63] The use of benzodiazepines (such as diazepam) to treat the pain is not recommended as it does not appear to help and is associated with risks.[66] Analgesics, other than NSAIDs, offer lesser, but some benefit with respect to pain whilst not causing the same level of gastrointestinal irritation.[3]
Lifestyle
Regular exercise is recommended as both safe and useful to maintain muscles strength and overall physical function.[67] It is uncertain if specific dietary measures have an effect.[68] Physical activity is beneficial for persons with Rheumatoid arthritis complaining of fatigue.[69] Occupational therapy has a positive role to play in improving functional ability of persons with rheumatoid arthritis.[70]
Disease modifying agents
Disease-modifying antirheumatic drugs (DMARDs) are the primary treatment for RA.[3] They are a diverse collection of drugs, grouped by use and convention. They have been found to improve symptoms, decrease joint damage, and improve overall functional abilities.[3] DMARDs should be started early in the disease as they result in disease remission in approximately half of people and improved outcomes overall.[71] The following drugs are considered as DMARDs: methotrexate, hydroxychloroquine, sulfasalazine, leflunomide, TNF-alpha inhibitors (certolizumab, infliximab and etanercept), abatacept, and anakinra. Rituximab and tocilizumab are monoclonal antibodies and are also DMARDs.
The most commonly used agent is methotrexate with other frequently used agents including sulfasalazine and leflunomide. Sodium aurothiomalate (gold) and cyclosporin are less commonly used due to more common adverse effects. Agents may be used in combinations.[3] Methotrexate is the most important and useful DMARD and is usually the first treatment.[64][65][72] Adverse effects should be monitored regularly with toxicity including gastrointestinal, hematologic, pulmonary, and hepatic.[72] Side effects such as nausea, vomiting or abdominal pain can be reduced by taking folic acid.[73] The most common undesirable effect is that it increases liver enzymes in almost 15% of people.[72] It is thus recommended that those who consistently demonstrate abnormal levels of liver enzymes or have a history of liver disease or alcohol use undergo liver biopsies.[74]
Biological agents should generally only be used if methotrexate and other conventional agents are not effective after a trial of three months.[75] They are associated with a higher rate of serious infections as compared to other DMARDs.[76] These agents used to treat rheumatoid arthritis include: tumor necrosis factor alpha (TNFα) blockers[3] such as infliximab; interleukin 1 blockers such as anakinra, monoclonal antibodies against B cells such as rituximab and tocilizumab,[77] T cell costimulation blocker such as abatacept among others. They are often used in combination with either methotrexate or leflunomide.[3] In those who are well controlled on TNF blockers decreasing the dose does not appear to affect overall function.[78] Persons should be screened for latent tuberculosis before starting any TNF blockers therapy to avoid reactivation.[14]
TNF blockers and methotrexate appear to have similar effectiveness when used alone and better results are obtained when used together. TNF blockers appear to have equivalent effectiveness with etanercept appearing to be the safest.[79] Abatacept appears effective for RA with 20% more people improving with treatment than without but long term safety studies are yet unavailable.[80] However, there is a lack of evidence to distinguish between the biologics available for RA.[81] Issues with the biologics include their high cost and association with infections including tuberculosis.[3]
Anti-inflammatory agents
NSAIDs reduce both pain and stiffness in those with RA.[3] Generally they appear to have no effect on people's long term disease course and thus are no longer first line agents.[3][82] NSAIDs should be used with caution in those with gastrointestinal, cardiovascular, or kidney problems.[83][84][85] Use of methotrexate together with NSAIDS is safe, if adequate monitoring is done.[86]
COX-2 inhibitors, such as celecoxib, and NSAIDs are equally effective.[87] They have a similar gastrointestinal risk as an NSAIDs plus a proton pump inhibitor.[88] In the elderly there is less gastrointestinal intolerance to celecoxib than to NSAIDs alone.[89] There however is an increased risk of myocardial infarction with COX-2 inhibitors.[87] Anti-ulcer medications are not recommended routinely but only in those high risk of gastrointestinal problems.[90]
Glucocorticoids can be used in the short term for flare-ups, while waiting for slow-onset drugs to take effect.[3] Injection of glucocorticoids into individual joints is also effective.[3] While long-term use reduces joint damage it also results in osteoporosis and susceptibility to infections, and thus is not recommended.[3]
Surgery
In early phases of the disease, an arthroscopic or open synovectomy may be performed. It consists of the removal of the inflamed synovia and prevents a quick destruction of the affected joints. Severely affected joints may require joint replacement surgery, such as knee replacement.[3] Postoperatively, physiotherapy is always necessary.
Alternative medicine
In general, there is not enough evidence to support any complementary health approaches for RA, with safety concerns for some of them. Some mind and body practices and dietary supplements may help people with symptoms and therefore may be beneficial additions to conventional treatments, but there is not enough evidence to draw conclusions.[6] A systematic review of CAM modalities (excluding fish oil) found that " The available evidence does not support their current use in the management of RA.".[91] Studies showing beneficial effects in RA on a wide variety of CAM modalities are often affected by publication bias and are generally not high quality evidence such as randomized controlled trials (RCTs).[5]
A 2005 Cochrane review states that low level laser therapy can be tried to improve pain and morning stiffness due to rheumatoid arthritis as there are few side-effects.[92]
There is some evidence that Tai Chi improves the range of motion of a joint in persons with rheumatoid arthritis.[93] The evidence for acupuncture is inconclusive[94] with it appearing to be equivalent to sham acupuncture.[95]
Dietary supplements
- Omega-3
- Some evidence supports omega-3 fatty acids and gamma-linolenic acid in RA.[96] The benefit from omega-3 appears modest but consistent,[97] though the current evidence is not strong enough to determine that supplementation with omega-3 polyunsaturated fatty acids (found in fish oil) is an effective treatment for RA.[98] Gamma-linolenic acid, which may reduce pain, tender joint count and stiffness, is generally safe.[99]
- Herbal
- The American College of Rheumatology states that no herbal medicines have health claims supported by high-quality evidence and thus they do not recommend their use.[100] There is no scientific basis to suggest that herbal supplements advertised as "natural" are safer for use than conventional medications as both are chemicals. Herbal medications, although labelled "natural", may be toxic or fatal if consumed.[100]
Due to the false belief that herbal supplements are always safe, there is sometimes a hesitancy to report their use which may increase the risk of adverse reaction.[5]
The following are under investigation for treatments for RA, based on preliminary promising results (not recommended for clinical use yet): boswellic acid,[101] curcumin,[102] devil's claw,[103][104] Euonymus alatus,[105] and thunder god vine (Tripterygium wilfordii).[106] NCCIH has noted that, "In particular, the herb thunder god vine (Tripterygium wilfordii) can have serious side effects."[6]
There is conflicting evidence on the role of erythropoiesis-stimulating agents for treatment of anemia in persons with rheumatoid arthritis.[107]
Pregnancy
More than 75% of people with rheumatoid arthritis have symptoms improve during pregnancy but might have worsenings after delivery.[14] Methotrexate and leflunomide are teratogenic (harmful to foetus) and not used in pregnancy. It is recommended women of childbearing age should use contraceptives to avoid pregnancy and to discontinue its use if pregnancy is planned.[64][72] Low dose of prednisolone, hydroxychloroquine and sulfasalazine are considered safe in pregnant persons with rheumatoid arthritis.
Vaccinations
People with RA have an increased risk of infections and mortality and recommended vaccinations can reduce these risks.[108] The inactivated influenza vaccine should be received annually.[109] The pneumococcal vaccine should be administered twice for people under the age 65 and once for those over 65.[110] Lastly, the live-attenuated zoster vaccine should be administered once after the age 60, but is not recommended in people on a tumor necrosis factor alpha blocker.[111]
Prognosis
The course of the disease varies greatly. Some people have mild short-term symptoms, but in most the disease is progressive for life. Around 20%–30% will have subcutaneous nodules (known as rheumatoid nodules); this is associated with a poor prognosis.
Prognostic factors
Poor prognostic factors include,
- Persistent synovitis
- Early erosive disease
- Extra-articular findings (including subcutaneous rheumatoid nodules)
- Positive serum RF findings
- Positive serum anti-CCP autoantibodies
- Carriership of HLA-DR4 "Shared Epitope" alleles
- Family history of RA
- Poor functional status
- Socioeconomic factors
- Elevated acute phase response (erythrocyte sedimentation rate [ESR], C-reactive protein [CRP])
- Increased clinical severity.
Mortality
RA reduces lifespan on average from three to twelve years.[113] Positive responses to treatment may indicate a better prognosis. A 2005 study by the Mayo Clinic noted that RA sufferers suffer a doubled risk of heart disease,[114] independent of other risk factors such as diabetes, alcohol abuse, and elevated cholesterol, blood pressure and body mass index. The mechanism by which RA causes this increased risk remains unknown; the presence of chronic inflammation has been proposed as a contributing factor.[115] It is possible that the use of new biologic drug therapies extend the lifespan of people with RA and reduce the risk and progression of atherosclerosis.[116] This is based on cohort and registry studies, and still remains hypothetical. It is still uncertain whether biologics improve vascular function in RA or not. There was an increase in total cholesterol and HDLc levels and no improvement of the atherogenic index.[117]
Epidemiology
RA affects between 0.5 and 1% of adults in the developed world with between 5 and 50 per 100,000 people newly developing the condition each year.[3] In 2010 it resulted in about 49,000 deaths globally.[118]
Onset is uncommon under the age of 15 and from then on the incidence rises with age until the age of 80. Women are affected three to five times as often as men.[14]
The age at which the disease most commonly starts is in women between 40 and 50 years of age, and for men somewhat later.[119] RA is a chronic disease, and although rarely, a spontaneous remission may occur, the natural course is almost invariably one of the persistent symptoms, waxing and waning in intensity, and a progressive deterioration of joint structures leading to deformations and disability.
History
The first known traces of arthritis date back at least as far as 4500 BC. A text dated 123 AD first describes symptoms very similar to RA. It was noted in skeletal remains of Native Americans found in Tennessee.[120] In Europe, the disease is vanishingly rare before the 17th century.[121] The first recognized description of RA in modern medicine was in 1800 by the French physician Dr Augustin Jacob Landré-Beauvais (1772–1840) who was based in the famed Salpêtrière Hospital in Paris.[8] The name "rheumatoid arthritis" itself was coined in 1859 by British rheumatologist Dr Alfred Baring Garrod.[122]
An anomaly has been noticed from the investigation of Pre-Columbian bones. The bones from the Tennessee site show no signs of tuberculosis even though it was prevalent at the time throughout the Americas.[123]
The art of Peter Paul Rubens may possibly depict the effects of RA. In his later paintings, his rendered hands show, in the opinion of some physicians, increasing deformity consistent with the symptoms of the disease.[124][125] RA appears to some to have been depicted in 16th-century paintings.[126] However, it is generally recognized in art historical circles that the painting of hands in the 16th and 17th century followed certain stylized conventions, most clearly seen in the Mannerist movement. It was conventional, for instance, to show the upheld right hand of Christ in what now appears a deformed posture. These conventions are easily misinterpreted as portrayals of disease.
Historic treatments for RA have also included: rest, ice, compression and elevation, apple diet, nutmeg, some light exercise every now and then, nettles, bee venom, copper bracelets, rhubarb diet, extractions of teeth, fasting, honey, vitamins, insulin, magnets, and electroconvulsive therapy (ECT).[127] The Prosorba column blood filtering device (removing IgG) was approved by the FDA in 1999 for treatment of RA[128] However it was discontinued at the end of 2006.[129]
Etymology
Rheumatoid arthritis is derived from the Greek word ῥεύμα-rheuma (nom.), ῥεύματος-rheumatos (gen.) ("flow, current"). The suffix -oid ("resembling") gives the translation as joint inflammation that resembles rheumatic fever. Rhuma which means watery discharge might refer to the fact that the joints are swollen or that the disease may be made worse by wet weather.[9]
References
- 1 2 3 4 5 6 "Handout on Health: Rheumatoid Arthritis". National Institute of Arthritis and Musculoskeletal and Skin Diseases. August 2014. Retrieved July 2, 2015.
- 1 2 3 4 Majithia V, Geraci SA (2007). "Rheumatoid arthritis: diagnosis and management". Am. J. Med. 120 (11): 936–9. doi:10.1016/j.amjmed.2007.04.005. PMID 17976416.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Scott DL, Wolfe F, Huizinga TW (Sep 25, 2010). "Rheumatoid arthritis". Lancet. 376 (9746): 1094–108. doi:10.1016/S0140-6736(10)60826-4. PMID 20870100.
- ↑ Singh, JA; Wells, GA; Christensen, R; Tanjong Ghogomu, E; Maxwell, L; Macdonald, JK; Filippini, G; Skoetz, N; Francis, D; Lopes, LC; Guyatt, GH; Schmitt, J; La Mantia, L; Weberschock, T; Roos, JF; Siebert, H; Hershan, S; Lunn, MP; Tugwell, P; Buchbinder, R (16 February 2011). "Adverse effects of biologics: a network meta-analysis and Cochrane overview.". The Cochrane database of systematic reviews (2): CD008794. doi:10.1002/14651858.CD008794.pub2. PMID 21328309.
- 1 2 3 Efthimiou P, Kukar M (2010). "Complementary and alternative medicine use in rheumatoid arthritis: proposed mechanism of action and efficacy of commonly used modalities". Rheumatology international. 30 (5): 571–86. doi:10.1007/s00296-009-1206-y. PMID 19876631.
- 1 2 3 "Rheumatoid Arthritis and Complementary Health Approaches". National Center for Complementary and Integrative Health. Retrieved July 1, 2015.
- ↑ GBD 2013 Mortality and Causes of Death, Collaborators (17 December 2014). "Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013.". Lancet. 385 (9963): 117–71. doi:10.1016/S0140-6736(14)61682-2. PMC 4340604
 . PMID 25530442.
. PMID 25530442. - 1 2 Landré-Beauvais AJ (1800). La goutte asthénique primitive (doctoral thesis). Paris. reproduced in Landré-Beauvais AJ (2001). "The first description of rheumatoid arthritis. Unabridged text of the doctoral dissertation presented in 1800". Joint Bone spine. 68 (2): 130–43. doi:10.1016/S1297-319X(00)00247-5. PMID 11324929.
- 1 2 Paget, Stephen A.; Lockshin, Michael D.; Loebl, Suzanne (2002). The Hospital for Special Surgery Rheumatoid Arthritis Handbook Everything You Need to Know. New York: John Wiley & Sons. p. 32. ISBN 9780471223344.
- ↑ Turesson C, O'Fallon WM, Crowson CS, Gabriel SE, Matteson EL (2003). "Extra-articular disease manifestations in rheumatoid arthritis: incidence trends and risk factors over 46 years". Ann. Rheum. Dis. 62 (8): 722–7. doi:10.1136/ard.62.8.722. PMC 1754626
 . PMID 12860726.
. PMID 12860726. - 1 2 3 Nicki R. Colledge, Brian R. Walker, Stuart H. Ralston, eds. (2010). Davidson's principles and practice of medicine. (21st ed.). Edinburgh: Churchill Livingstone/Elsevier. ISBN 978-0-7020-3084-0.
- ↑ "An approach to Early Arthritis". Pn.lifehugger.com. 12 January 2009. Archived from the original on May 27, 2010.
- ↑ Gaffo A, Saag KG, Curtis JR (2006). "Treatment of rheumatoid arthritis". Am J Health Syst Pharm. 63 (24): 2451–2465. doi:10.2146/ajhp050514. PMID 17158693.
- 1 2 3 4 5 6 7 8 9 Shah, Ankur. Harrison's Principle of Internal Medicine (18th ed.). United States: McGraw Hill. p. 2738. ISBN 978-0-07174889-6.
- 1 2 Turesson, C (May 2013). "Extra-articular rheumatoid arthritis.". Current opinion in rheumatology. 25 (3): 360–6. doi:10.1097/bor.0b013e32835f693f. PMID 23425964.
- ↑ "Rheumatoid Lung Disease – What Is Rheumatoid Lung Disease?". Arthritis.about.com. February 27, 2011. Retrieved March 3, 2011.
- ↑ de Groot K (August 2007). "[Renal manifestations in rheumatic diseases]". Internist (Berl). 48 (8): 779–85. doi:10.1007/s00108-007-1887-9. PMID 17571244.
- ↑ Robbins, Stanley Leonard; Kumar, Vinay; Abbas, Abdul K.; Cotran, Ramzi S.; Fausto, Nelson (2010). Vinay Kumar, Abul K. Abbas, Nelson Fausto, eds. Robbins and Cotran pathologic basis of disease. Robbins Pathology Series. Elsevier. p. 205. ISBN 978-1-4160-3121-5.
- ↑ Wolfe F, Mitchell DM, Sibley JT, Fries JF, Bloch DA, Williams CA, Spitz PW, Haga M, Kleinheksel SM, Cathey MA (April 1994). "The mortality of rheumatoid arthritis". Arthritis Rheum. 37 (4): 481–94. doi:10.1002/art.1780370408. PMID 8147925.
- ↑ Aviña-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D (2008). "Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies". Arthritis Rheum. 59 (12): 1690–1697. doi:10.1002/art.24092. PMID 19035419.
- 1 2 Gupta A, Fomberstein B (2009). "Evaluating cardiovascular risk in rheumatoid arthritis". Journal of Musculoskeletal Medicine. 26 (8): 481–94.
- 1 2 Selmi, Carlo; Santis, Maria De; Gershwin, M Eric (2011). "Liver involvement in subjects with rheumatic disease". Arthritis Research & Therapy. BioMed Central. 13 (3): 226. doi:10.1186/ar3319. PMID 21722332.
- ↑ Citation: H. Rehman : Hemolytic Anemia following Mycoplasma Infection. The Internet Journal of Hematology. 2008 Volume 4 Number 1
- ↑ Baecklund E, Iliadou A, Askling J, Ekbom A, Backlin C, Granath F, Catrina AI, Rosenquist R, Feltelius N, Sundström C, Klareskog L (2006). "Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis". Arthritis & Rheumatism. 54 (3): 692–701. doi:10.1002/art.21675. PMID 16508929.
- ↑ Franklin J, Lunt M, Bunn D, Symmons D, Silman A (2006). "Incidence of lymphoma in a large primary care derived cohort of cases of inflammatory polyarthritis". Annals of the Rheumatic Diseases. 65 (5): 617–622. doi:10.1136/ard.2005.044784. PMC 1798140
 . PMID 16249224.
. PMID 16249224. - ↑ Edwards JC, Cambridge G, Abrahams VM (1999). "Do self-perpetuating B lymphocytes drive human autoimmune disease?". Immunology. 97 (2): 188–96. doi:10.1046/j.1365-2567.1999.00772.x. PMC 2326840
 . PMID 10447731.
. PMID 10447731. - ↑ Plenge RM, Seielstad M, Padyukov L, Lee AT, Remmers EF, Ding B, Liew A, Khalili H, Chandrasekaran A, Davies LR, Li W, Tan AK, Bonnard C, Ong RT, Thalamuthu A, Pettersson S, Liu C, Tian C, Chen WV, Carulli JP, Beckman EM, Altshuler D, Alfredsson L, Criswell LA, Amos CI, Seldin MF, Kastner DL, Klareskog L, Gregersen PK (20 September 2007). "TRAF1–C5 as a Risk Locus for Rheumatoid Arthritis — A Genomewide Study". The New England Journal of Medicine. 357 (12): 1199–209. doi:10.1056/NEJMoa073491. PMC 2636867
 . PMID 17804836.
. PMID 17804836. - ↑ Goeldner I, Skare TL, de Messias Reason IT, Nisihara RM, Silva MB, Utiyama SR (Aug 2010). "Anti-cyclic citrullinated peptide antibodies and rheumatoid factor in rheumatoid arthritis patients and relatives from Brazil". Rheumatology (Oxford). 49 (8): 1590–3. doi:10.1093/rheumatology/keq134. PMID 20457731.
- ↑ "The Genetics Behind Rheumatoid Arthritis". Arthritis Foundation. Retrieved December 17, 2012.
- ↑ Silman AJ, MacGregor AJ, Thomson W, Holligan S, Carthy D, Farhan A, Ollier WE (1993). "Twin concordance rates for rheumatoid arthritis: Results from a nationwide study". British journal of rheumatology. 32 (10): 903–907. doi:10.1093/rheumatology/32.10.903. PMID 8402000.
- ↑ Bellamy N, Duffy D, Martin N, Mathews J (1992). "Rheumatoid arthritis in twins: A study of aetiopathogenesis based on the Australian Twin Registry". Annals of the rheumatic diseases. 51 (5): 588–593. doi:10.1136/ard.51.5.588. PMC 1005687
 . PMID 1616321.
. PMID 1616321. - 1 2 Sugiyama D, Nishimura K, Tamaki K, Tsuji G, Nakazawa T, Morinobu A, Kumagai S (2010). "Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies". Ann. Rheum. Dis. 69 (1): 70–81. doi:10.1136/ard.2008.096487. PMID 19174392.
- ↑ Liao KP, Alfredsson L, Karlson EW (May 2009). "Environmental influences on risk for rheumatoid arthritis". Current Opinion in Rheumatology. 21 (3): 279–83. doi:10.1097/BOR.0b013e32832a2e16. PMC 2898190
 . PMID 19318947.
. PMID 19318947. - ↑ Alvarez-Lafuente R, Fernández-Gutiérrez B, de Miguel S, Jover JA, Rollin R, Loza E, Clemente D, Lamas JR (September 2005). "Potential relationship between herpes viruses and rheumatoid arthritis: analysis with quantitative real time polymerase chain reaction". Ann. Rheum. Dis. 64 (9): 1357–9. doi:10.1136/ard.2004.033514. PMC 1755640
 . PMID 16100341.
. PMID 16100341. - ↑ Balandraud N, Roudier J, Roudier C (2004). "Epstein-Barr virus and rheumatoid arthritis". Autoimmun Rev. 3 (5): 362–7. doi:10.1016/j.autrev.2004.02.002. PMID 15288002.
- ↑ Gatenby P, Lucas R, Swaminathan A (2013). "Vitamin D deficiency and risk for rheumatic diseases: an update". Curr Opin Rheumatol. 25 (2): 184–91. doi:10.1097/BOR.0b013e32835cfc16. PMID 23370372.
- 1 2 Wen H, Baker JF (March 2011). "Vitamin D, immunoregulation, and rheumatoid arthritis". Journal of Clinical Rheumatology. 17 (2): 102–7. doi:10.1097/RHU.0b013e31820edd18. PMID 21364350.
- ↑ Guillot X, Semerano L, Saidenberg-Kermanac'h N, Falgarone G, Boissier MC (2010). "Vitamin D and inflammation". Joint Bone Spine. 77 (6): 552–7. doi:10.1016/j.jbspin.2010.09.018. PMID 21067953.
- ↑ St-Arnaud R. "The direct role of vitamin D on bone homeostasis". doi:10.1016/j.abb.2008.03.038. PMID 18424254.
- 1 2 Doherty, M; Lanyon, P; Ralston, SH. Musculosketal Disorders-Davidson's Principle of Internal Medicine (20th ed.). Elsevier. pp. 1100–1106.
- 1 2 3 Plenge RM, Seielstad M, Padyukov L, Lee AT, Remmers EF, Ding B, Liew A, Khalili H, Chandrasekaran A, Davies LR, Li W, Tan AK, Bonnard C, Ong RT, Thalamuthu A, Pettersson S, Liu C, Tian C, Chen WV, Carulli JP, Beckman EM, Altshuler D, Alfredsson L, Criswell LA, Amos CI, Seldin MF, Kastner DL, Klareskog L, Gregersen PK (2007). "TRAF1-C5 as a Risk Locus for Rheumatoid Arthritis — A Genomewide Study". N. Engl. J. Med. 357 (12): 1199–209. doi:10.1056/NEJMoa073491. PMC 2636867
 . PMID 17804836.
. PMID 17804836. - ↑ Padyukov L, Silva C, Stolt P, Alfredsson L, Klareskog L (2004). "A gene-environment interaction between smoking and shared epitope genes in HLA-DR provides a high risk of seropositive rheumatoid arthritis". Arthritis Rheum. 50 (10): 3085–92. doi:10.1002/art.20553. PMID 15476204.
- 1 2 3 Maverakis E, Kim K, Shimoda M, Gershwin M, Patel F, Wilken R, Raychaudhuri S, Ruhaak LR, Lebrilla CB (2015). "Glycans in the immune system and The Altered Glycan Theory of Autoimmunity". J Autoimmun. 57 (6): 1–13. doi:10.1016/j.jaut.2014.12.002. PMC 4340844
 . PMID 25578468.
. PMID 25578468. - ↑ Boldt AB, Goeldner I, de Messias-Reason IJ. "Relevance of the lectin pathway of complement in rheumatic diseases". doi:10.1016/B978-0-12-394317-0.00012-1. PMID 22397030.
- ↑ "HBP310 Immunology". SUNY Stony Brook Pathology Department. Archived from the original on August 7, 2006. Retrieved September 20, 2008.
- ↑ Ghaffar, Abdul. "Hypersensitivity States". University of South Carolina School of Medicine. Retrieved May 29, 2016.
- ↑ Holmes, N. (1999). "Lecture 14: Hypersensitivity". Immunology Division, Department of Pathology, University of Cambridge. Archived from the original on February 6, 2006. Retrieved September 20, 2008.
- ↑ Schueller-Weidekamm C. Modern ultrasound methods yield stronger arthritis work-up. Diagnostic Imaging. May 2010:20–22.
- ↑ Westwood OM, Nelson PN, Hay FC (2006). "Rheumatoid factors: what's new?". Rheumatology (Oxford). 45 (4): 379–85. doi:10.1093/rheumatology/kei228. PMID 16418203.
- 1 2 Nishimura K, Sugiyama D, Kogata Y, Tsuji G, Nakazawa T, Kawano S, Saigo K, Morinobu A, Koshiba M, Kuntz KM, Kamae I, Kumagai S (2007). "Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis". Ann. Intern. Med. 146 (11): 797–808. doi:10.7326/0003-4819-146-11-200706050-00008. PMID 17548411.
- ↑ Nishimura K; Sugiyama D; Kogata Y; Tsuji, G; Nakazawa, T; Kawano, S; Saigo, K; Morinobu, A; et al. (2007). "Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis". Ann. Intern. Med. 146 (11): 797–808. doi:10.7326/0003-4819-146-11-200706050-00008. PMID 17548411.
- ↑ Renger F, Bang H, Fredenhagen G, Natusch A, Backhaus M, Feist E, Egerer K, Burmester GR. "Anti-MCV Antibody Test for the Diagnosis of Rheumatoid Arthritis Using a POCT-Immunoassay". American College of Rheumatology, 2008 Annual Scientific Meeting, poster presentation.
- ↑ Luime JJ, Colin EM, Hazes JM, Lubberts E (2009). "Does anti-MCV has additional value as serological marker in the diagnostic and prognostic work-up of patients with rheumatoid arthritis? A systematic review". Ann Rheum Dis. 69 (2): 337–44. doi:10.1136/ard.2008.103283. PMID 19289382.
- 1 2 Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Ménard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovsky J, Wolfe F, Hawker G (2010). "2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative" (PDF). Ann. Rheum. Dis. 69 (9): 1580–8. doi:10.1136/ard.2010.138461. PMID 20699241. Archived from the original (PDF) on August 21, 2010.
- ↑ Aletaha, Daniel; Neogi, Tuhina; Silman, Alan J.; Funovits, Julia; Felson, David T.; Bingham, Clifton O.; Birnbaum, Neal S.; Burmester, Gerd R.; Bykerk, Vivian P.; Cohen, Marc D.; Combe, Bernard; Costenbader, Karen H.; Dougados, Maxime; Emery, Paul; Ferraccioli, Gianfranco; Hazes, Johanna M. W.; Hobbs, Kathryn; Huizinga, Tom W. J.; Kavanaugh, Arthur; Kay, Jonathan; Kvien, Tore K.; Laing, Timothy; Mease, Philip; Ménard, Henri A.; Moreland, Larry W.; Naden, Raymond L.; Pincus, Theodore; Smolen, Josef S.; Stanislawska-Biernat, Ewa; Symmons, Deborah; Tak, Paul P.; Upchurch, Katherine S.; Vencovský, Jiří; Wolfe, Frederick; Hawker, Gillian. "2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative". Arthritis & Rheumatism. 62 (9): 2569–2581. doi:10.1002/art.27584.
- ↑ Arnett F, Edworthy S, Bloch D, McShane D, Fries J, Cooper N, Healey L, Kaplan S, Liang M, Luthra H (1988). "The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis" (PDF). Arthritis Rheum. 31 (3): 315–24. doi:10.1002/art.1780310302. PMID 3358796. Archived from the original (PDF) on January 24, 2011. Retrieved February 8, 2011.
- 1 2 Berkow R, ed. (1992). The Merck Manual (16th ed.). Merck Publishing Group. pp. 1307–08. ISBN 0-911910-16-6.
- ↑ Lovy MR, Starkebaum G, Uberoi S (1996). "Hepatitis C infection presenting with rheumatic manifestations: a mimic of rheumatoid arthritis". J. Rheumatol. 23 (6): 1238–9. PMID 8782126.
- ↑ Kelly, Janis (22 February 2005) DAS28 not always a reliable indicator of treatment effect in RA, Medscape Medical News.
- 1 2 Prevoo ML, van 't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL (1995). "Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis". Arthritis Rheum. 38 (1): 44–8. doi:10.1002/art.1780380107. PMID 7818570.
- ↑ Yazici, Yusuf (2013). "Tools for monitoring remission in rheumatoid arthritis: any will do, let's just pick one and start measuring" (PDF). Arthritis Research & Therapy. 15: 104. doi:10.1186/ar4139. Retrieved October 20, 2014.
- ↑ Spriggs, Brenda B. "Rheumatoid Arthritis Prevention". Healthline Networks. Retrieved September 16, 2014.
- 1 2 Saag KG, Teng GG, Patkar NM, et al. (2008). "American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis". Arthritis Rheum. 59 (6): 762–84. doi:10.1002/art.23721. PMID 18512708.
- 1 2 3 Amy M. Wasserman (2011). "Diagnosis and Management of Rheumatoid Arthritis". American Family Physician. 84 (11): 1245–1252. PMID 22150658.
- 1 2 Chris Deighton; Rachel O’Mahony; Jonathan Tosh; Claire Turner; Michael Rudolf; Guideline Development Group (2009). "Management of rheumatoid arthritis: summary of NICE guidance". British Medical Journal. 338: 710–712. doi:10.1136/bmj.b702.
- ↑ Richards BL, Whittle SL, Buchbinder R (Jan 18, 2012). Richards, Bethan L, ed. "Muscle relaxants for pain management in rheumatoid arthritis". Cochrane database of systematic reviews (Online). 1: CD008922. doi:10.1002/14651858.CD008922.pub2. PMID 22258993.
- ↑ Hurkmans E, van der Giesen FJ, Vliet Vlieland TP, Schoones J, Van den Ende EC (Oct 7, 2009). Hurkmans, Emalie, ed. "Dynamic exercise programs (aerobic capacity and/or muscle strength training) in patients with rheumatoid arthritis". Cochrane database of systematic reviews (Online) (4): CD006853. doi:10.1002/14651858.CD006853.pub2. PMID 19821388.
- ↑ Hagen KB, Byfuglien MG, Falzon L, Olsen SU, Smedslund G (Jan 21, 2009). Hagen, Kåre Birger, ed. "Dietary interventions for rheumatoid arthritis". Cochrane database of systematic reviews (Online) (1): CD006400. doi:10.1002/14651858.CD006400.pub2. PMID 19160281.
- ↑ Cramp, Fiona (2013). "Non-pharmacological interventions for fatigue in rheumatoid arthritis.". Cochrane Database of Systematic Reviews (8): Art. No.: CD008322. doi:10.1002/14651858.CD008322.pub2.
- ↑ Steultjens, Esther EMJ (2004). "Occupational therapy for rheumatoid arthritis". Cochrane Database of Systematic Reviews (1). doi:10.1002/14651858.CD003114.pub2.
- ↑ Gramling A, O'Dell JR (2012). "Initial management of rheumatoid arthritis". Rheum. Dis. Clin. North Am. 38 (2): 311–25. doi:10.1016/j.rdc.2012.05.003. PMID 22819086.
- 1 2 3 4 DiPiro, Joseph T., Robert L. Talbert, Gary C. Yee, Gary R. Matzke, Barbara G. Wells, and L. Michael Posey (2008) Pharmacotherapy: a pathophysiologic approach. 7th ed. New York: McGraw-Hill, ISBN 978-0-07-147899-1.
- ↑ Shea, B.; Swinden, M.V.; Tanjong Ghogomu, E.; Ortiz, Z.; Katchamart, W.; Rader, T.; Bombardier, C.; Wells, George A; Tugwell, Peter (May 31, 2013). "Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis". The Cochrane database of systematic reviews. 5 (5): CD000951. doi:10.1002/14651858.CD000951.pub2. PMID 23728635.
- ↑ American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines (2002). "Guidelines for the management of rheumatoid arthritis: 2002 Update". Arthritis & Rheumatism. 46 (2): 328–346. doi:10.1002/art.10148.
- ↑ Singh, JA; Furst, DE; Bharat, A; Curtis, JR; Kavanaugh, AF; Kremer, JM; Moreland, LW; O'Dell, J; Winthrop, KL; Beukelman, T; Bridges SL, Jr; Chatham, WW; Paulus, HE; Suarez-Almazor, M; Bombardier, C; Dougados, M; Khanna, D; King, CM; Leong, AL; Matteson, EL; Schousboe, JT; Moynihan, E; Kolba, KS; Jain, A; Volkmann, ER; Agrawal, H; Bae, S; Mudano, AS; Patkar, NM; Saag, KG (May 2012). "2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis.". Arthritis Care & Research. 64 (5): 625–39. doi:10.1002/acr.21641. PMID 22473917.
- ↑ Singh, Jasvinder A; Cameron, Chris; Noorbaloochi, Shahrzad; Cullis, Tyler; Tucker, Matthew; Christensen, Robin; Ghogomu, Elizabeth Tanjong; Coyle, Doug; Clifford, Tammy; Tugwell, Peter; Wells, George A (May 2015). "Risk of serious infection in biological treatment of patients with rheumatoid arthritis: a systematic review and meta-analysis". The Lancet. 386 (9990): 258–265. doi:10.1016/S0140-6736(14)61704-9.
- ↑ Edwards J, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close D, Stevens R, Shaw T; Szczepanski; Szechinski; Filipowicz-Sosnowska; Emery; Close; Stevens; Shaw (2004). "Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis". N Engl J Med. 350 (25): 2572–81. doi:10.1056/NEJMoa032534. PMID 15201414.
- ↑ van Herwaarden, N; den Broeder, AA; Jacobs, W; van der Maas, A; Bijlsma, JW; van Vollenhoven, RF; van den Bemt, BH (Sep 29, 2014). "Down-titration and discontinuation strategies of tumor necrosis factor-blocking agents for rheumatoid arthritis in patients with low disease activity". The Cochrane database of systematic reviews. 9 (9): CD010455. doi:10.1002/14651858.CD010455.pub2. PMID 25264908.
- ↑ Aaltonen KJ, Virkki LM, Malmivaara A, Konttinen YT, Nordström DC, Blom M; Virkki; Malmivaara; Konttinen; Nordström; Blom (2012). Hernandez, Adrian V, ed. "Systematic review and meta-analysis of the efficacy and safety of existing TNF blocking agents in treatment of rheumatoid arthritis". PLoS ONE. 7 (1): e30275. doi:10.1371/journal.pone.0030275. PMC 3260264
 . PMID 22272322.
. PMID 22272322. - ↑ Maxwell L, Singh JA; Singh (Oct 7, 2009). Maxwell, Lara, ed. "Abatacept for rheumatoid arthritis". Cochrane database of systematic reviews (Online) (4): CD007277. doi:10.1002/14651858.CD007277.pub2. PMID 19821401.
- ↑ Singh JA, Christensen R, Wells GA, Suarez-Almazor ME, Buchbinder R, Lopez-Olivo MA, Tanjong Ghogomu E, Tugwell P; Christensen; Wells; Suarez-Almazor; Buchbinder; Lopez-Olivo; Tanjong Ghogomu; Tugwell (Oct 7, 2009). Singh, Jasvinder A, ed. "Biologics for rheumatoid arthritis: an overview of Cochrane reviews". Cochrane database of systematic reviews (Online) (4): CD007848. doi:10.1002/14651858.CD007848.pub2. PMID 19821440.
- ↑ Tarp S, Bartels EM, Bliddal H, Furst DE, Boers M, Danneskiold-Samsøe B, Rasmussen M, Christensen R; Bartels; Bliddal; Furst; Boers; Danneskiold-Samsøe; Rasmussen; Christensen (November 2012). "Effect of nonsteroidal antiinflammatory drugs on the C-reactive protein level in rheumatoid arthritis: a meta-analysis of randomized controlled trials". Arthritis and rheumatism. 64 (11): 3511–21. doi:10.1002/art.34644. PMID 22833186.
- ↑ Radner H, Ramiro S, Buchbinder R, Landewé RB, van der Heijde D, Aletaha D; Ramiro; Buchbinder; Landewé; Van Der Heijde; Aletaha (Jan 18, 2012). Radner, Helga, ed. "Pain management for inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and other spondylarthritis) and gastrointestinal or liver comorbidity". Cochrane database of systematic reviews (Online). 1: CD008951. doi:10.1002/14651858.CD008951.pub2. PMID 22258995.
- ↑ McCormack PL (2011). "Celecoxib: a review of its use for symptomatic relief in the treatment of osteoarthritis, rheumatoid arthritis and ankylosing spondylitis". Drugs. 71 (18): 2457–89. doi:10.2165/11208240-000000000-00000. PMID 22141388.
- ↑ Marks JL, Colebatch AN, Buchbinder R, Edwards CJ; Colebatch; Buchbinder; Edwards (Oct 5, 2011). Marks, Jonathan L, ed. "Pain management for rheumatoid arthritis and cardiovascular or renal comorbidity". Cochrane database of systematic reviews (Online) (10): CD008952. doi:10.1002/14651858.CD008952.pub2. PMID 21975789.
- ↑ Colebatch, AN; Marks, Jonathan L; Edwards, Christopher J (2011). "Safety of non-steroidal anti-inflammatory drugs, including aspirin and paracetamol (acetaminophen) in people receiving methotrexate for inflammatory arthritis (rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, other spondyloarthritis". Cochrane Database of Systematic Reviews. doi:10.1002/14651858.CD008872.pub2.
- 1 2 Chen YF, Jobanputra P, Barton P, Bryan S, Fry-Smith A, Harris G, Taylor RS; Jobanputra; Barton; Bryan; Fry-Smith; Harris; Taylor (April 2008). "Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation". Health technology assessment (Winchester, England). 12 (11): 1–278, iii. doi:10.3310/hta12110. PMID 18405470.
- ↑ Wang X, Tian HJ, Yang HK, Wanyan P, Peng YJ; Tian; Yang; Wanyan; Peng (October 2011). "Meta-analysis: cyclooxygenase-2 inhibitors are no better than nonselective nonsteroidal anti-inflammatory drugs with proton pump inhibitors in regard to gastrointestinal adverse events in osteoarthritis and rheumatoid arthritis". European journal of gastroenterology & hepatology. 23 (10): 876–80. doi:10.1097/MEG.0b013e328349de81. PMID 21900785.
- ↑ Mallen SR, Essex MN, Zhang R; Essex; Zhang (July 2011). "Gastrointestinal tolerability of NSAIDs in elderly patients: a pooled analysis of 21 randomized clinical trials with celecoxib and nonselective NSAIDs". Current medical research and opinion. 27 (7): 1359–66. doi:10.1185/03007995.2011.581274. PMID 21561397.
- ↑ "Nonsteroidal anti-inflammatory drugs: add an anti-ulcer drug for patients at high risk only. Always limit the dose and duration of treatment with NSAIDs". Prescrire Int. 20 (119): 216–9. 2011. PMID 21954519.
- ↑ Macfarlane GJ, El-Metwally A, De Silva V, Ernst E, Dowds GL, Moots RJ; El-Metwally; De Silva; Ernst; Dowds; Moots; Arthritis Research UK Working Group on Complementary Alternative Medicines (2011). "Evidence for the efficacy of complementary and alternative medicines in the management of rheumatoid arthritis: a systematic review". Rheumatology (Oxford). 50 (9): 1672–83. doi:10.1093/rheumatology/ker119. PMID 21652584.
- ↑ Brosseau L, Robinson V, Wells G, Debie R, Gam A, Harman K, Morin M, Shea B, Tugwell P (2005). "Low level laser therapy (Classes I, II and III) for treating rheumatoid arthritis". Cochrane Database Syst Rev. 4 (4): CD002049. doi:10.1002/14651858.CD002049.pub2. PMID 16235295.
- ↑ Han, Alcie; Judd, Maria; Welch, Vivian; Wu, Taixiang; Tugwell, Peter; Wells, George A (2004). "Tai chi for treating rheumatoid arthritis". Cochrane Database of Systematic Reviews (3). doi:10.1002/14651858.CD004849.
- ↑ Lee MS, Shin B-C, Ernst E; Shin; Ernst (2008). "Acupuncture for rheumatoid arthritis: a systematic review". Rheumatology. 47 (12): 1747–53. doi:10.1093/rheumatology/ken330. PMID 18710899.
- ↑ Macfarlane GJ, Paudyal P, Doherty M, Ernst E, Lewith G, MacPherson H, Sim J, Jones GT; Paudyal; Doherty; Ernst; Lewith; MacPherson; Sim; Jones; Arthritis Research UK Working Group on Complementary Alternative Therapies for the Management of the Rheumatic Diseases (2012). "A systematic review of evidence for the effectiveness of practitioner-based complementary and alternative therapies in the management of rheumatic diseases: rheumatoid arthritis". Rheumatology. 51 (9): 1707–13. doi:10.1093/rheumatology/kes133. PMID 22661556.
- ↑ Pirotta, M (September 2010). "Arthritis disease – the use of complementary therapies". Australian family physician. 39 (9): 638–40. PMID 20877766.
- ↑ Miles EA, Calder PC; Calder (June 2012). "Influence of marine n-3 polyunsaturated fatty acids on immune function and a systematic review of their effects on clinical outcomes in rheumatoid arthritis". The British journal of nutrition. 107 Suppl 2 (S2): S171–84. doi:10.1017/S0007114512001560. PMID 22591891.
- ↑ Ruggiero C, Lattanzio F, Lauretani F, Gasperini B, Andres-Lacueva C, Cherubini A; Lattanzio; Lauretani; Gasperini; Andres-Lacueva; Cherubini (2009). "Omega-3 polyunsaturated fatty acids and immune-mediated diseases: inflammatory bowel disease and rheumatoid arthritis" (PDF). Current pharmaceutical design. 15 (36): 4135–48. doi:10.2174/138161209789909746. PMID 20041815.
- ↑ Soeken, K L; Miller, S A; Ernst, E. "Herbal medicines for the treatment of rheumatoid arthritis: a systematic review". Centre for Reviews and Dissemination. National Institute for Health Research. Retrieved March 23, 2013.
- 1 2 "Herbal Remedies, Supplements and Acupuncture for Arthritis". American College of Rheumatology. Retrieved May 3, 2013.
- ↑ Abdel-Tawab M, Werz O, Schubert-Zsilavecz M; Werz; Schubert-Zsilavecz (June 2011). "Boswellia serrata: an overall assessment of in vitro, preclinical, pharmacokinetic and clinical data". Clinical pharmacokinetics. 50 (6): 349–69. doi:10.2165/11586800-000000000-00000. PMID 21553931.
- ↑ White B, Judkins DZ; Judkins (March 2011). "Clinical Inquiry. Does turmeric relieve inflammatory conditions?". The Journal of family practice. 60 (3): 155–6. PMID 21369559.
- ↑ Wegener, T. (1999). "Therapy of degenerative diseases of the musculoskeletal system with South African devil's claw (Harpagophytum procumbens DC)". Wiener medizinische Wochenschrift (1946). 149 (8–10): 254–257. PMID 10483693.
- ↑ Denner SS (2007). "A review of the efficacy and safety of devil's claw for pain associated with degenerative musculoskeletal diseases, rheumatoid, and osteoarthritis". Holist Nurs Pract. 21 (4): 203–7. doi:10.1097/01.HNP.0000280932.65581.72. PMID 17627199.
- ↑ Zhang LF, Zhao JX; Zhao (December 2005). "The recent research situation of Euonymus alatus". Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica. 30 (24): 1895–8. PMID 16494017.
- ↑ Bao J, Dai SM; Dai (2011). "A Chinese herb Tripterygium wilfordii Hook F in the treatment of rheumatoid arthritis: mechanism, efficacy, and safety". Rheumatol. Int. 31 (9): 1123–9. doi:10.1007/s00296-011-1841-y. PMID 21365177.
- ↑ Martí-Carvajal, Arturo J; Agreda-Pérez, Luis H; Solà, Ivan; Simancas-Racines, Daniel (2013). "Erythropoiesis-stimulating agents for anemia in rheumatoid arthritis". Cochrane Database of Systematic Reviews. doi:10.1002/14651858.CD000332.pub3.
- ↑ Perry, Lisa M.; Winthrop, Kevin L.; Curtis, Jeffrey R. (13 June 2014). "Vaccinations for Rheumatoid Arthritis". Current Rheumatology Reports. 16 (8). doi:10.1007/s11926-014-0431-x.
- ↑ Grohskopf, LA; Olsen, SJ; Sokolow, LZ; Bresee, JS; Cox, NJ; Broder, KR; Karron, RA; Walter, EB; Centers for Disease Control and, Prevention (15 August 2014). "Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) – United States, 2014–15 influenza season". MMWR. Morbidity and mortality weekly report. 63 (32): 691–7. PMID 25121712.
- ↑ Black, CL; Yue, X; Ball, SW; Donahue, SM; Izrael, D; de Perio, MA; Laney, AS; Lindley, MC; Graitcer, SB; Lu, PJ; Williams, WW; Bridges, CB; DiSogra, C; Sokolowski, J; Walker, DK; Greby, SM (19 September 2014). "Influenza vaccination coverage among health care personnel – United States, 2013–14 influenza season.". MMWR. Morbidity and mortality weekly report. 63 (37): 805–11. PMID 25233281.
- ↑ Hales, CM; Harpaz, R; Ortega-Sanchez, I; Bialek, SR; Centers for Disease Control and Prevention, (CDC) (22 August 2014). "Update on recommendations for use of herpes zoster vaccine.". MMWR. Morbidity and mortality weekly report. 63 (33): 729–31. PMID 25144544.
- ↑ "WHO Disease and injury country estimates". World Health Organization. 2009. Retrieved November 11, 2009.
- ↑ Kitas, George (4 April 2006) Why is life span shortened by Rheumatoid Arthritis? National Rheumatoid Arthritis Society
- ↑ Rheumatoid Arthritis Patients Have Double the Risk of Heart Failure. mayoclinic.org (3 February 2005).
- ↑ "Cardiac disease in rheumatoid arthritis". Johns Hopkins University. 2002. Archived from the original on October 9, 2006.
- ↑ Atzeni F, Turiel M, Caporali R, Cavagna L, Tomasoni L, Sitia S, Sarzi-Puttini P; Turiel; Caporali; Cavagna; Tomasoni; Sitia; Sarzi-Puttini (2010). "The effect of pharmacological therapy on the cardiovascular system of patients with systemic rheumatic diseases". Autoimmun Rev. 9 (12): 835–9. doi:10.1016/j.autrev.2010.07.018. PMID 20678592.
- ↑ Damjanov, N; Nurmohamed, MT; Szekanecz, Z (Mar 18, 2014). "Biologics, cardiovascular effects and cancer.". BMC medicine. 12 (1): 48. doi:10.1186/1741-7015-12-48. PMC 3984692
 . PMID 24642038.
. PMID 24642038. - ↑ Lozano, 1R; Naghavi, M; Foreman, K; Lim, S; Shibuya, K; Aboyans, V; Abraham, J; Adair, T; et al. (Dec 15, 2012). "Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010". Lancet. 380 (9859): 2095–128. doi:10.1016/S0140-6736(12)61728-0. PMID 23245604.
- ↑ Alamanos Y, Voulgari PV, Drosos AA; Voulgari; Drosos (2006). "Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review". Semin. Arthritis Rheum. 36 (3): 182–8. doi:10.1016/j.semarthrit.2006.08.006. PMID 17045630.
- ↑ Rothschild, Bruce M. "Tennessee Origins of Rheumatoid Arthritis". Mcclungmuseum.utk.edu. Archived from the original on February 2, 2012. Retrieved March 3, 2011.
- ↑ "Bones of contention". Arthritis Research UK. April 1999. Archived from the original on February 19, 2003. Retrieved February 5, 2013.
- ↑ Garrod AB (1859). The Nature and Treatment of Gout and Rheumatic Gout. London: Walton and Maberly.
- ↑ Rothschild BM, Rothschild C, Helbling M; Rothschild; Helbling (2003). "Unified theory of the origins of erosive arthritis: conditioning as a protective/directing mechanism?". J. Rheumatol. 30 (10): 2095–102. PMID 14528501.
- ↑ Appelboom T, de Boelpaepe C, Ehrlich GE, Famaey JP; De Boelpaepe; Ehrlich; Famaey (1981). "Rubens and the question of antiquity of rheumatoid arthritis". JAMA. 245 (5): 483–6. doi:10.1001/jama.245.5.483. PMID 7005475.
- ↑ Kelly, Janis (14 June 2005). "Did RA travel from New World to Old? The Rubens connection". Medscape. Retrieved March 3, 2011.
- ↑ Dequeker J.; Rico H. (1992). "Rheumatoid arthritis-like deformities in an early 16th-century painting of the Flemish-Dutch school". JAMA. 268 (2): 249–251. doi:10.1001/jama.268.2.249. PMID 1608144.
- ↑ Hart FD (1976). "History of the treatment of rheumatoid arthritis". Br Med J. 1 (6012): 763–5. doi:10.1136/bmj.1.6012.763. PMC 1639217
 . PMID 177148.
. PMID 177148. - ↑ Fresenius HemoCare, Inc., "New Hope for Rheumatoid Arthritis Patients," press release, September 17, 1999.
- ↑ "Prosorba Column – Which Rheumatoid Arthritis Patients Are Good Candidates for the Prosorba Column?". Arthritis.about.com. Retrieved March 3, 2011.
External links
| Wikimedia Commons has media related to Rheumatoid arthritis. |
- Rheumatoid arthritis at DMOZ
- Charles Weber. "History of rheumatoid arthritis".
- Singh, JA; Saag, KG; Bridges SL, Jr; Akl, EA; Bannuru, RR; Sullivan, MC; Vaysbrot, E; McNaughton, C; Osani, M; Shmerling, RH; Curtis, JR; Furst, DE; Parks, D; Kavanaugh, A; O'Dell, J; King, C; Leong, A; Matteson, EL; Schousboe, JT; Drevlow, B; Ginsberg, S; Grober, J; St Clair, EW; Tindall, E; Miller, AS; McAlindon, T (January 2016). "2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis.". Arthritis & rheumatology (Hoboken, N.J.). 68 (1): 1–26. doi:10.1002/art.39480. PMID 26545940.