Superalloy

A superalloy, or high-performance alloy, is an alloy that exhibits several key characteristics: excellent mechanical strength, resistance to thermal creep deformation, good surface stability, and resistance to corrosion or oxidation. The crystal structure is typically face-centered cubic austenitic. Examples of such alloys are Hastelloy, Inconel, Waspaloy, Rene alloys, Haynes alloys, Incoloy, MP98T, TMS alloys, and CMSX single crystal alloys.
Superalloy development has relied heavily on both chemical and process innovations. Superalloys develop high temperature strength through solid solution strengthening. An important strengthening mechanism is precipitation strengthening which forms secondary phase precipitates such as gamma prime and carbides. Oxidation or corrosion resistance is provided by elements such as aluminium and chromium.
The primary application for such alloys is in turbine engines, both aerospace and marine.
Chemical development
Because these alloys are intended for high temperature applications (i.e. holding their shape at temperatures near their melting point) their creep and oxidation resistance are of primary importance. Nickel (Ni) based superalloys have emerged as the material of choice for these applications.[1] The properties of these Ni based superalloys can be tailored to a certain extent through the addition of many other elements, both common and exotic, including not only metals, but also metalloids and nonmetals; chromium, iron, cobalt, molybdenum, tungsten, tantalum, aluminium, titanium, zirconium, niobium, rhenium, yttrium, vanadium, carbon, boron or hafnium are some examples of the alloying additions used. Each of these additions has been chosen to serve a particular purpose in optimizing the properties for high temperature application.
Creep resistance is dependent on slowing the speed of dislocation motion within a crystal structure. In modern Ni based superalloys, the γ’-Ni3(Al,Ti) phase present acts as a barrier to dislocation motion. For this reason, this γ’ intermetallic phase, when present in high volume fractions, drastically increases the strength of these alloys due to its ordered nature and high coherency with the γ matrix. The chemical additions of aluminum and titanium promote the creation of the γ’ phase. The γ’ phase size can be precisely controlled by careful precipitation strengthening heat treatments. Many superalloys are produced using a two-phase heat treatment that creates a dispersion of cuboidal γ’ particles known as the primary phase, with a fine dispersion between these known as secondary γ’. In order to improve the oxidation resistance of these alloys, Al, Cr, B, and Y are added. The Al and Cr form oxide layers that passivate the surface and protect the superalloy from further oxidation while B and Y are used to improve the adhesion of this oxide scale to the substrate.[2] Cr, Fe, Co, Mo and Re all preferentially partition to the γ matrix while Al, Ti, Nb, Ta, and V preferentially partition to the γ’ precipitates and solid solution strengthen the matrix and precipitates respectively. In addition to solid solution strengthening, if grain boundaries are present, certain elements are chosen for grain boundary strengthening. B and Zr tend to segregate to the grain boundaries which reduces the grain boundary energy and results in better grain boundary cohesion and ductility.[3] Another form of grain boundary strengthening is achieved through the addition of C and a carbide former, such as Cr, Mo, W, Nb, Ta, Ti, or Hf, which drives precipitation of carbides at grain boundaries and thereby reduces grain boundary sliding.
While Ni based superalloys are excellent high temperature materials and have proven very useful, Co based superalloys potentially possess superior hot corrosion, oxidation, and wear resistance as compared to Ni-based superalloys. For this reason, efforts have also been put into developing Co based superalloys over the past several years. Despite that, traditional Co based superalloys have not found widespread usage because they have a lower strength at high temperature than Ni based superalloys.[4] The main reason for this is that they appear to lack the γ’ precipitation strengthening that is so important in the high temperature strength of Ni-based superalloys. A 2006 report on metastable γ’-Co3(Al,W) intermetallic compound with the L12 structure suggests Co based alloys as alternative to traditional Ni based superalloys. However this class of alloys was reported in a PhD thesis by C.S. Lee in 1971.[5] The two-phase microstructure consists of cuboidal γ’ precipitates embedded in a continuous γ matrix and is therefore morphologically identical to the microstructure observed in Ni based superalloys. Like in the Ni-based system, there is a high degree of coherency between the two phases which is one of the main factors resulting in the superior strength at high temperatures. This provides a pathway for the development of a new class of load-bearing Co based superalloys for application in severe environments.[6] In these alloys 'W' is the crucial addition for getting γ’ intermetallic compound that makes them much denser (>9.6 gm/cm3) compared to Ni-based superalloys. Recently a new class of γ - γ’ cobalt based superalloys have been developed that are "W" free and have much lower density comparable to nickel based superalloys.[7][8][9] In addition to the fact that many of the properties of these new Co based superalloys could be better than those of the more traditional Ni based ones, Co also has a higher melting temperature than Ni. Therefore, if the high temperature strength could be improved, the development of novel Co based superalloys could allow for an increase in jet engine operation temperature resulting in an increased efficiency.
Metallurgy of superalloys
Ni-based superalloy phases
- Gamma (γ): This phase composes the matrix of Ni-based superalloy. It is a solid solution fcc austenitic phase of the alloying elements.[10][11] Alloying elements found in most commercial Ni-based alloys are, C, Cr, Mo, W, Nb, Fe, Ti, Al, V, and Ta. During the formation of these materials, as the Ni-alloys are cooled from the melt, carbides begin to precipitate, at even lower temperatures γ'phase precipitates.[10][12]
- Gamma Prime (γ'): This phase constitutes the precipitate used to strengthen the alloy. It is an intermetallic phase based on Ni3(Ti,Al) which have an ordered FCC L12 structure.[11] The γ' phase is coherent with the matrix of the superalloy having a lattice parameter that varies by around 0.5%. Ni (Ti,Al) are ordered systems with Ni atoms at the cube faces and either Al or Ti atoms at the cube edges As particles of γ' precipitates aggregate, they decrease their energy states by aligning along the <100> directions forming cuboidal structures.[10] This phase has a window of instability between 600 °C and 850 °C, inside of which γ' will transform into the HCP η phase. For applications at temperatures below 650 °C, the γ" phase can be utilized for strengthening.[13]
- Gamma Double Prime (γ"): This phase typically possesses the composition of Ni3Nb or Ni3V and is used to strengthen Ni-based superalloys at lower temperatures (<650 °C) relative to γ'. The crystal structure of γ" is body-centered tetragonal (BCT), and the phase precipitates as 60 nm by 10 nm discs with the (001) planes in γ" parallel to the {001} family in γ. These anisotropic discs form as a result of lattice mismatch between the BCT precipitate and the FCC matrix. This lattice mismatch leads to high coherency strains which, together with order hardening, comprise the primary strengthening mechanisms. The γ" phase is unstable above approximately 650 °C.[13]
- Carbide Phases: Carbide formation is usually considered deleterious although in Ni-based superalloys they are used to stabilize the structure of the material against deformation at high temperatures. Carbides form at the grain boundaries inhibiting grain boundary motion.[10][11]
- Topologically Close-Packed (TCP) Phases: The term "TCP Phase" refers to any member of a family of phases (including the σ phase, the χ phase, the μ phase, and the Laves phase) which are not atomically close-packed but possess some close-packed planes with HCP stacking. TCP phases are characterized by their tendency to be highly brittle and deplete the γ matrix of strengthening, solid solution refractory elements (including Cr, Co, W, and Mo). These phases form as a result of kinetics after long periods of time (thousands of hours) at high temperatures (>750 °C).[13]
Co-based superalloy phases
- Gamma (γ): Similar to Ni-based superalloys, this is the phase of the superalloy’s matrix. While not used commercially to the extent of Ni-based superalloys, alloying elements found in research Co-based alloys are C, Cr, W, Ni, Ti, Al, Ir, and Ta.[4][14] Chromium is also used in Cobalt based superalloys (occasionally up to 20 wt.%) as it provides oxidation and corrosion resistance, critical for material use in gas turbines.[15]
- Gamma Prime (γ'): Just as in Ni-based super alloys, this phase constitutes the precipitate used to strengthen the alloy. In this case, it is usually close packed with a L12 structure of Co3Ti or fcc Co3T, though both W and Al have been found to integrate into these cuboidal precipitates quite well. The elements Ta, Nb, and Ti integrate into the γ’ phase and are quite effective at stabilizing it at high temperatures. This stabilization is quite important as the lack of stability is one of the key factors that makes Co-based superalloys weaker than their Ni-base cousins at elevated temperatures.[4][16]
- Carbide Phases: As is common with carbide formation, its appearance in Co-based superalloys does provide precipitation hardening, but does decrease low-temperature ductility.[14]
Microstructure of superalloys
In pure Ni3Al phase atoms of aluminium are placed at the vertices of the cubic cell and form the sublattice A. Atoms of nickel are located at centers of the faces and form the sublattice B. The phase is not strictly stoichiometric. There may exist an excess of vacancies in one of the sublattices, which leads to deviations from stoichiometry. Sublattices A and B of the γ'-phase can solute a considerable proportion of other elements. The alloying elements are dissolved in the γ-phase as well. The γ'-phase hardens the alloy through an unusual mechanism called the yield strength anomaly. Dislocations dissociate in the γ'-phase, leading to the formation of an anti-phase boundary. At elevated temperature, the free energy associated with the anti-phase boundary (APB) is considerably reduced if it lies on a particular plane, which by coincidence is not a permitted slip plane. One set of partial dislocations bounding the APB cross-slips so that the APB lies on the low-energy plane, and, since this low-energy plane is not a permitted slip plane, the dissociated dislocation is now effectively locked. By this mechanism, the yield strength of γ'-phase Ni3Al actually increases with temperature up to about 1000 °C, giving superalloys their currently unrivalled high-temperature strength.
Initial material selection for blade applications in Gas Turbine engines included alloys like the Nimonic series alloys in the 1940s.[17] The early Nimonic series incorporated γ' Ni3(Al,Ti) precipitates in a γ matrix, as well as various metal-carbon carbides (e.g. Cr23C6) at the grain boundaries[18] for additional grain boundary strength. Turbine blade components were forged until vacuum induction casting technologies were introduced in the 1950s.[17] This process significantly improved cleanliness, reduced defects, and increased the strength and temperature capability of the material.
Modern superalloys were developed in the 1980s. The first generation superalloys incorporated increased aluminium, titanium, tantalum, and niobium content in order to increase the γ' volume fraction in these alloys. Examples of first generation superalloys include: PWA1480, René N4 and SRR99. Additionally, the volume fraction of the γ' precipitates increased to about 50-70%. With the advent of single crystal, or monocrystal, solidification techniques (see Bridgman technique) for superalloys that enable grain boundaries to be entirely eliminated from a casting. Because the material contained no grain boundaries, carbides were unnecessary as grain boundary strengthers and were thus eliminated.[17]
The second and third generation superalloys introduced about 3 and 6 weight per cent Rhenium, for increased temperature capability. Re is a slow diffuser and typically partitions to the γ matrix, decreasing the rate of diffusion (and thereby high temperature creep) and improving high temperature performance and increasing service temperatures by 30 °C and 60 °C in second and third generation superalloys, respectively.[19] Re has also been shown to promote the formation of rafts of the γ' phase (as opposed to cuboidal precipitates). The presence of rafts can decrease creep rate in the power-law regime (controlled by dislocation climb), but can also potentially increase the creep rate if the dominant mechanism is particle shearing. Furthermore, Re tends to promote the formation of brittle TCP phases, which has led to the strategy of reducing Co, W, Mo, and particularly Cr. Younger generations of Ni-based superalloys have significantly reduced Cr content for this reason, however with the reduction in Cr comes a reduction in oxidation resistance. Advanced coating techniques are now used to offset the loss of oxidation resistance accompanying the decreased Cr contents.[13][20] Examples of second generation superalloys include PWA1484, CMSX-4 and René N5. Third generation alloys include CMSX-10, and René N6. Fourth, Fifth, and even Sixth generation superalloys have been developed which incorporate Ruthenium additions, making them more expensive still than the prior generation's Re-containing alloys. The effect of Ru on the promotion of TCP phases is not well-determined. Early reports determined that Ru decreased the supersaturation of Re in the matrix and thereby diminished the susceptibility to TCP phase formation.[21] More recent studies have noted the opposite effect. Chen, et al., found that in two alloys differing significantly only in Ru content (USTB-F3 and USTB-F6) that the addition of Ru increased both the partitioning ratio as well as the supersaturation in the γ matrix of Cr and Re, and thereby promoted the formation of TCP phases.[22]
The current trend is to avoid very expensive and very heavy elements. An example is Eglin steel, a budget material with compromised temperature range and chemical resistance. It does not contain rhenium or ruthenium and its nickel content is limited. To reduce fabrication costs, it was chemically designed to melt in a ladle (though with improved properties in a vacuum crucible). Also, conventional welding and casting is possible before heat-treatment. The original purpose was to produce high-performance, inexpensive bomb casings, but the material has proven widely applicable to structural applications, including armor.
Single-crystal superalloys
Single-crystal superalloys (SX or SC superalloys) are formed as a single crystal using a modified version of the directional solidification technique, so there are no grain boundaries in the material. The mechanical properties of most other alloys depend on the presence of grain boundaries, but at high temperatures, they would participate in creep and must be replaced by other mechanisms. In many such alloys, islands of an ordered intermetallic phase sit in a matrix of disordered phase, all with the same crystalline lattice. This approximates the dislocation-pinning behavior of grain boundaries, without introducing any amorphous solid into the structure.
Single crystal (SX) superalloys have wide application in the high pressure turbine section of aero and industrial gas turbine engines due to the unique combination of properties and performance. Since introduction of single crystal casting technology, SX alloy development has focused on increased temperature capability, and major improvements in alloy performance have been associated with the introduction of new alloying elements, including rhenium (Re) and ruthenium (Ru).[23]
With increasing turbine entry temperature, it is important to gain a fundamental understanding of the physical phenomena occurring during creep deformation of single crystal superalloys under such extreme condition (i.e. high temperature and high stress). The creep deformation behavior of superalloy single crystal is strongly temperature, stress, orientation and alloy dependent. For a single-crystal superalloy, there are 3 different mode of creep deformation under regimes of different temperature and stress: Rafting, Tertiary and Primary.[24] At low temperature (~750 °C), SX alloys exhibits mostly primary creep behavior. Matan et al. concluded that the extent of primary creep deformation depends strongly on the angle between the tensile axis and the <001>/<011> symmetry boundary.[25] At temperature above 850 °C, tertiary creep dominates and promotes strain softening behavior.[26] When temperature exceeds 1000 °C, the rafting effect is prevalent where cubic particles transform into flat shapes under tensile stress [27] The rafts would also form perpendicular to the tensile axis, since γ phase was transported out of the vertical channels and into the horizontal ones. After conducting unaxial creep deformation of <001> orientated CMSX-4 single crystal superalloy at 1105 °C and 100 MPa, Reed et al. has established that rafting is beneficial to creep life since it delays evolution of creep strain. In addition, rafting would occur quickly and suppress the accumulation of creep strain until a critical strain is reached.[28]
Oxidation in superalloys
For superalloys operating at high temperatures and exposed to corrosive environments, the oxidation behavior is of paramount concern. Oxidation involves chemical reactions of the alloying elements with oxygen to form new oxide phases, generally at the surface of the metal. If unmitigated, oxidation can degrade the alloy over time in a variety of ways, including:[29][30]
- sequential oxidation, cracking, and spalling of the surface, leading to erosion of the alloy over time.
- embrittlement of the surface through the introduction of oxide phases, promoting crack formation and fatigue failure
- depletion of key alloying elements, affecting the mechanical properties of the superalloy and possibly compromising its performance.
The primary strategy used to limit these deleterious processes is called selective oxidation. Simply, the alloy is designed such that the ratio of alloying elements promotes formation of a specific oxide phase that can then act as a barrier to further oxidation. Most commonly, aluminum and chromium are used in this role, because they form relatively thin and continuous oxide layers of alumina (Al2O3) and chromia (Cr2O3), respectively. Furthermore, they possess low oxygen diffusivities, effectively halting further oxidation beneath this layer. In the ideal case, oxidation proceeds through 2 stages. First, transient oxidation involves the conversion of various elements, especially the majority elements (e.g. nickel or cobalt). Transient oxidation proceeds until the selective oxidation of the sacrificial element forms a complete barrier layer.[29]
The protective effect of selective oxidation can be undermined by numerous mechanisms. The continuity of the thin sacrificial oxide layer can be compromised by mechanical disruption due to stress or may be disrupted as a result of the kinetics of oxidation (e.g. if diffusion of oxygen is too fast). If the layer is not continuous, its effectiveness as a diffusion barrier to oxygen is significantly reduced. The stability of the oxide layer is also strongly influenced by the presence of other minority elements. For example, the addition of boron, silicon, and yttrium to superalloys promotes oxide layer adhesion, reducing spalling and maintaining the integrity of the protective oxide layer.[31]
It should be noted that oxidation is only the most basic form of chemical degradation superalloys may experience. More complex corrosion processes are common when operating environments include salts and sulfur compounds, or under chemical conditions that change dramatically over time. These issues and those of basic oxidation are often also addressed through thin coatings.
Superalloy processing
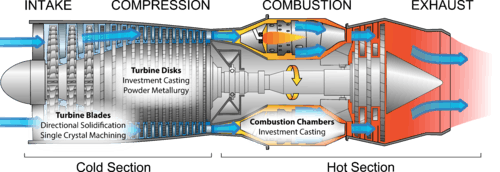
The historical developments in superalloy processing have brought about considerable increases in superalloy operating temperatures. Superalloys were originally iron based and cold wrought prior to the 1940s. In the 1940s investment casting of cobalt base alloys significantly raised operating temperatures. The development of vacuum melting in the 1950s allowed for very fine control of the chemical composition of superalloys and reduction in contamination and in turn led to a revolution in processing techniques such as directional solidification of alloys and single crystal superalloys.[32]
There are many forms of superalloy present within a gas turbine engine, and processing methods vary widely depending on the necessary properties of each specific part.
Casting and forging
Casting and forging are traditional metallurgical processing techniques that can be used to generate both polycrystalline and monocrystalline products. Polycrystalline casts tend to have higher fracture resistance, while monocrystalline casts have higher creep resistance.
Jet turbine engines employ both poly and mono crystalline components to take advantage of their individual strengths. The disks of the high pressure turbine, which are near the central hub of the engine are polycrystalline, The turbine blades, which extend radially into the engine housing, experience a much greater centripetal force, necessitating creep resistance. As a result, turbine blades are typically monocrystalline or polycrystalline with a preferred crystal orientation.
Investment casting
Investment casting is a metallurgical processing technique in which a wax form is fabricated and used as a template for a ceramic mold. Briefly, a ceramic mold is poured around the wax form, the wax form is melted out of the ceramic mold, and molten metal is poured into the void left by the wax. This leads to a metal form in the same shape as the original wax form. Investment casting leads to a polycrystalline final product, as nucleation and growth of crystal grains occurs at numerous locations throughout the solid matrix. Generally, the polycrystalline product has no preferred grain orientation.
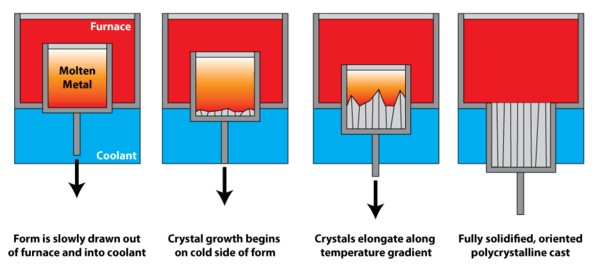
Directional solidification
Directional solidification uses a thermal gradient to promote nucleation of metal grains on a low temperature surface, as well as to promote their growth along the temperature gradient. This leads to grains elongated along the temperature gradient, and significantly greater creep resistance parallel to the long grain direction. In polycrystalline turbine blades, directional solidification is used to orient the grains parallel to the centripetal force.
Single crystal growth
Single crystal growth starts with a seed crystal which is used to template growth of a larger crystal. The overall process is lengthy, and additional processing via machining is necessary after the single crystal is grown.
Powder metallurgy
Powder metallurgy is a class of modern processing techniques in which metals are first converted into a powder form, and then formed into the desired shape by heating below the melting point. This is in contrast to casting, which occurs with molten metal. Superalloy manufacturing often employs powder metallurgy because of its material efficiency - typically much less waste metal must be machined away from the final product - and its ability to facilitate mechanical alloying. Mechanical alloying is a process by which reinforcing particles are incorporated into the superalloy matrix material by repeated fracture and welding.[33]
Sintering and hot isostatic pressing
Sintering and hot isostatic pressing are processing techniques used to densify materials from a loosely packed "green body" into a solid object with physically merged grains. Sintering occurs below the melting point, and causes adjacent particles to merge at their boundaries, leading to a strong bond between them. In hot isostatic pressing, a sintered material is placed in a pressure vessel and compressed from all directions (isostatically) in an inert atmosphere to affect densification.[34]
Additive manufacturing
Selective laser sintering is an additive manufacturing procedure used to create intricately detailed forms from a CAD file. In CAD, a shape is designed and the converted into slices. These slices are sent to a laser writer to print the final product. In brief, a bed of metal powder is prepared, and the first slice of the CAD design is formed in the powder bed by a high energy laser sintering the particles together. After this first slice is generated, the powder bed moves downwards, and a new batch of metal powder is rolled over the top of the slice. The second layer is then sintered with the laser, and the process is repeated until all the slices in the CAD file have been processed.[35]
Coating of superalloys
In modern gas turbine, the turbine entry temperature (~1750K) has exceeded the incipient melting temperature of superalloys (~1600K), with the help of surface engineering. Under such extreme working condition, the qualification of coating becomes vital.[36]
Different types of coating
Historically, three “generations” of coatings have been developed: diffusion coatings, overlay coatings and thermal barrier coatings. Diffusion coatings, mainly constituted with aluminide or platinum-aluminide, is still the most common form of surface protection. To further enhance resistance to corrosion and oxidation, MCrAlX-based overlay coatings (M=Ni or Co, X=Y, Hf, Si) are deposited to surface of superalloys. Compared to diffusion coatings, overlay coatings are less dependent on the composition of the substrate, but also more expensive, since they must be carried out by air or vacuum plasma spraying (APS/VPS) [37] or else electron beam physical vapour deposition (EB-PVD).[38] Thermal barrier coatings provide by far the best enhancement in working temperature and coating life. It is estimated that modern TBC of thickness 300 μm, if used in conjunction with a hollow component and cooling air, has the potential to lower metal surface temperatures by a few hundred degrees.[39]
Thermal barrier coatings
Thermal barrier coatings (TBCs) are used extensively on the surface of superalloy in both commercial and military gas turbine engines to increase component life and engine performance.[40] A coating of about 1-200 µm can reduce the temperature at the superalloy surface by up to 200K. TBCs are really a system of coatings consisting of a bond coat, a thermally grown oxide (TGO), and a thermally insulating ceramic top coat. In most applications, the bond coat is either a MCrAlY (where M=Ni or NiCo) or a Pt modified aluminide coating. A dense bond coat is required to provide protection of the superalloy substrate from oxidation and hot corrosion attack and to form an adherent, slow growing TGO on its surface. The TGO is formed by oxidation of the aluminum that is contained in the bond coat. The current (first generation) thermal insulation layer is composed of 7wt % yttria stabilized zirconia (7YSZ) with a typical thickness of 100-300 µm. Yttria stabilized zirconia is used due to its low thermal conductivity (2.6W/mK for fully dense material), relatively high coefficient of thermal expansion, and good high temperature stability. The electron beam directed vapor deposition (EB-DVD) process used to apply the TBC to turbine airfoils produces a columnar microstructure with several levels of porosity. The porosity between the columns is critical to providing strain tolerance (via a very low in-plane modulus), as it would otherwise spall on thermal cycling due to thermal expansion mismatch with the superalloy substrate. The porosity within the columns reduces the thermal conductivity of the coating.
Bond coat
The bond coat adheres the thermal barrier coating to the superalloy substrate. Additionally, the bond coat provides oxidation protection and functions as a diffusion barrier against the motion of substrate atoms towards the environment. There are five major types of bond coats, the aluminides, the platinum-aluminides, MCrAlY, cobalt-cermets, and nickel-chromium. For the aluminide bond coatings, the final composition and structure of the coating depends on the composition of the substrate. Aluminides also lack ductility below 750 °C, and exhibit a limited by thermomechanical fatigue strength. The Pt-aluminides are very similar to the aluminide bond coats except for a layer of Pt (5-10 μm) deposited on the blade. The Pt is believed to aid in oxide adhesion and contributes to hot corrosion. The cost of Pt plating is justified by the increased blade life span. The MCrAlY is the latest generation of bond coat and does not strongly interact with the substrate. Normally applied by plasma spraying, MCrAlY coatings are secondary aluminum oxide formers. This means that the coatings form an outer layer of chromium oxide (chromia), and a secondary aluminum oxide (alumina) layer underneath. These oxide formations occur at high temperatures in the range of those that superalloys usually encounter.[41] The chromia provides oxidation and hot-corrosion resistance. The alumina controls oxidation mechanisms by limiting oxide growth by self-passivating. The yttrium enhances the oxide adherence to the substrate, and limits the growth of grain boundaries (which can lead to flaking of the coating).[42] Investigation indicates that addition of rhenium and tantalum increases oxidation resistance. Cobalt-cermet based coatings consisting of materials such as tungsten carbide/cobalt can be used due to excellent resistance to abrasion, corrosion, erosion, and heat.[43] These cermet coatings perform well in situations where temperature and oxidation damage are significant concerns, such as boilers. One of the unique advantages of cobalt cermet coatings is a minimal loss of coating mass over time, due to the strength of carbides within the mixture. Overall, cermet coatings are useful in situations where mechanical demands are equal to chemical demands for superalloys. Nickel-chromium coatings are used most frequently in boilers fed by fossil fuels, electric furnaces, and waste incineration furnaces, where the danger of oxidizing agents and corrosive compounds in the vapor must be dealt with.[44] The specific method of spray-coating depends on the composition of the coatings. Nickel-chromium coatings that also contain iron or aluminum perform much better (in terms of corrosion resistance) when they are sprayed and laser glazed, while pure nickel-chromium coatings perform better when thermally sprayed exclusively.[45]
Process methods of coating
Superalloy products that are subjected to high working temperatures and corrosive atmosphere (such as high pressure turbine region of jet engines) are coated with various kinds of coating. Several kinds of coating process are applied: pack cementation process, gas phase coating (both are a type of chemical vapor deposition (CVD)), thermal spraying, and physical vapor deposition. In most cases, after the coating process near-surface regions of parts are enriched with aluminium, the matrix of the coating being nickel aluminide.
Pack cementation process
The pack cementation process is carried out at lower temperatures, about 750 °C. The parts are loaded into boxes that contain a mixture of powders: active coating material, containing aluminum, activator (chloride or fluoride), and thermal ballast, like aluminum oxide. At high temperatures the gaseous aluminum chloride is transferred to the surface of the part and diffuses inside (mostly inward diffusion). After the end of the process the so-called "green coating" is produced, which is too thin and brittle for direct use. A subsequent diffusion heat treatment (several hours at temperatures about 1080 °C) leads to further inward diffusion and formation of the desired coating.
Thermal spraying
Thermal spraying is a process of applying coatings by heating a feedstock of precursor material and spraying it on a surface. Different specific techniques are used depending on desired particle size, coat thickness, spray speed, desired area, etc.[46] The coatings applied by thermal spraying of any kind, however, rely on adhesion to the surface. As a result, the surface of the superalloy must be cleaned and prepared, usually polished, before application of the thermal coating.[47]
Plasma spraying
Of the various thermal spray methods, one of the more ideal and commonly used techniques for coating superalloys is plasma spraying. This is due to the versatility of usable coatings, and the high-temperature performance of plasma-sprayed coatings.[48] Plasma spraying can accommodate a very wide range of materials, much more so than other techniques. As long as the difference between melting and decomposition temperatures is greater than 300 Kelvin, a material can be melted and applied as a coating via plasma spraying.[49]
Gas phase coating
This process is carried out at higher temperatures, about 1080 °C. The coating material is usually loaded onto special trays without physical contact with the parts to be coated. The coating mixture contains active coating material and activator, but usually does not contain thermal ballast. As in the pack cementation process, the gaseous aluminium chloride (or fluoride) is transferred to the surface of the part. However, in this case the diffusion is outwards. This kind of coating also requires diffusion heat treatment.
Failure mechanisms in thermal barrier coating systems
Failure of thermal barrier coating usually manifests as delamination, which arises from the temperature gradient during thermal cycling between ambient temperature and working conditions coupled with the difference in thermal expansion coefficient of the substrate and coating. It is rare for the coating to fail completely – some pieces of it remain intact, and significant scatter is observed in the time to failure if testing is repeated under identical conditions.[1] There are various degradation mechanisms for thermal barrier coating,[50][51] and some or all of these must operate before failure finally occurs:
- Oxidation at the interface of thermal barrier coating and underlying bond coat;[52]
- The depletion of aluminum in bond coat due to oxidation [53] and diffusion with substrate;[54]
- Thermal stresses from mismatch in thermal expansion coefficient and growth stress due to the formation of thermally grown oxide layer;[55]
- Imperfections near thermally grown oxide layer;[56][57][58]
- Various other complicating factors during engine operation.[59][60][61][62][63]
Additionally, TBC life is very dependent upon the combination of materials (substrate, bond coat, ceramic) and processes (EB-PVD, plasma spraying) used.
Applications
Nickel-based superalloys are used in load-bearing structures to the highest homologous temperature of any common alloy system (Tm = 0.9, or 90% of their melting point). Among the most demanding applications for a structural material are those in the hot sections of turbine engines. The preeminence of superalloys is reflected in the fact that they currently comprise over 50% of the weight of advanced aircraft engines. The widespread use of superalloys in turbine engines coupled with the fact that the thermodynamic efficiency of turbine engines is increased with increasing turbine inlet temperatures has, in part, provided the motivation for increasing the maximum-use temperature of superalloys. In fact, during the past 30 years turbine airfoil temperature capability has increased on average by about 4 °F (2.2 °C) per year. Two major factors which have made this increase possible are
- Advanced processing techniques, which improved alloy cleanliness (thus improving reliability) and/or enabled the production of tailored microstructures such as directionally solidified or single-crystal material.
- Alloy development resulting in higher-use-temperature materials primarily through the additions of refractory elements such as Re, W, Ta, and Mo.
About 60% of the use-temperature increases have occurred due to advanced cooling concepts; 40% have resulted from material improvements. State-of-the-art turbine blade surface temperatures are near 2,100 °F (1,150 °C); the most severe combinations of stress and temperature corresponds to an average bulk metal temperature approaching 1,830 °F (1,000 °C).
Although superalloys retain significant strength to temperatures near 1,800 °F (980 °C), they tend to be susceptible to environmental attack because of the presence of reactive alloying elements (which provide their high-temperature strength). Surface attack includes oxidation, hot corrosion, and thermal fatigue. In the most demanding applications, such as turbine blade and vanes, superalloys are often coated to improve environmental resistance.[64]
Research and development of new superalloys
The availability of superalloys during past decades has led to a steady increase in the turbine entry temperatures and the trend is expected to continue. Sandia National Laboratories is studying a new method for making superalloys, known as radiolysis. It introduces an entirely new area of research into creating alloys and superalloys through nanoparticle synthesis. This process holds promise as a universal method of nanoparticle formation. By developing an understanding of the basic material science behind these nanoparticle formations, there is speculation that it might be possible to expand research into other aspects of superalloys.
There may be considerable disadvantages in making alloys by this method. About half of the use of superalloys is in applications where the service temperature is close to the melting temperature of the alloy. It is common therefore to use single crystals. The above method produces polycrystalline alloys, which suffer from an unacceptable level of creep.
Future paradigm in alloy development focus on reduction of weight, improving oxidation and corrosion resistance while maintaining the strength of the alloy. Furthermore, with the increasing demand for turbine blade for power generation, another focus of alloy design is to reduce the cost of super alloys.
See also
References
- 1 2 Reed, Roger C. The Superalloys: Fundamentals and Applications. Cambridge, UK: Cambridge UP, 2006.
- ↑ Klein, L., Y. Shen, M. S. Killian, and S. Virtanen. "Effect of B and Cr on the High Temperature Oxidation Behavior of Novel γ/γ′Strengthened Co-base Superalloys." Corrosion Science 53 (2011): 2713-720.
- ↑ Shinagawa, K., Toshihiro Omori, Katsunari Oikawa, Ryosuke Kainuma, and Kiyohito Ishida. "Ductility Enhancement by Boron Addition in Co–Al–W High-temperature Alloys." Scripta Materialia 61.6 (2009): 612-15.
- 1 2 3 Sato, J. "Cobalt-Base High-Temperature Alloys." Science 312.5770 (2006): 90-91.
- ↑ Lee, CS. "Precipitation-hardening characteristics of ternary cobalt - aluminum - X alloys".
- ↑ Suzuki, A., Garret C. DeNolf, and Tresa M. Pollock. "Flow Stress Anomalies in γ/γ′ Two-phase Co–Al–W-base Alloys" Scripta Materialia 56.5 (2007): 385-88.
- ↑ Makineni, S.K.; Nithin, B.; Chattopadhyay, K. (March 2015). "A new tungsten-free γ–γ' Co–Al–Mo–Nb-based superalloy". Scripta Materialia. 98: 36–39. doi:10.1016/j.scriptamat.2014.11.009.
- ↑ Makineni, S.K.; Nithin, B.; Chattopadhyay, K. (February 2015). "Synthesis of a new tungsten-free γ–γ′ cobalt-based superalloy by tuning alloying additions". Acta Materialia. 85: 85–94. doi:10.1016/j.actamat.2014.11.016.
- ↑ Makineni, S.K.; Samanta, A.; Rojhirunsakool, T.; Alam, T.; Nithin, B.; Singh, A.K.; Banerjee, R.; Chattopadhyay, K. (September 2015). "A new class of high strength high temperature Cobalt based γ–γ′ Co–Mo–Al alloys stabilized with Ta addition". Acta Materialia. 97: 29–40. doi:10.1016/j.actamat.2015.06.034.
- 1 2 3 4 Sabol, G. P. & Stickler, R. Microstructure of Nickel-Based Superalloys. physica status solidi (b) 35, 11-52, doi:10.1002/pssb.19690350102 (1969).
- 1 2 3 Randy Bowman, Superalloys: A Primer and History
- ↑ Minoru Doi et.al Gamma/Gamma-Prime Microstructure Formed by Phase Separation of Gamma-Prime Precipitates in Ni-Al-Ti Alloys
- 1 2 3 4 Dunand, David C. Materials Science & Engineering 435: High Temperature Materials. Northwestern University, Evanston. 25 Feb. 2016. Lecture.
- 1 2 Cui, C. "A New Co-Base Superalloy Strengthened by γ’ Phase." Materials Transactions 47.8 (2006): 2099-2102.
- ↑ Coutsouradis, D.; Davin, A.; Lamberigts, M. (April 1987). "Cobalt-based superalloys for applications in gas turbines". Materials Science and Engineering. 88: 11–19. doi:10.1016/0025-5416(87)90061-9.
- ↑ Suzuki, A., and Tresa M. Pollock. "High-temperature strength and deformation of γ/γ′ two-phase Co–Al–W-base alloys "Acta Materialia 56.6 (2008): 1288-97.
- 1 2 3 R.C. Reed, The Superalloys. Fundamentals and Applications
- ↑ D. Bombač, M. Fazarinc, G. Kugler, S. Spajić, Microstructure development of Nimonic 80A superalloys during hot deformation, Materials and Geoenvironment, 55:3 (2008) 319-328.
- ↑ Reed, R.C. The Superalloys: Fundamentals and Applications. Cambridge: Cambridge University Press; 2006. 121.
- ↑ Dunand, David C. "High-Temperature Materials for Energy Conversion" Materials Science & Engineering 381: Materials for Energy-Efficient Technology. Northwestern University, Evanston. 3 Feb. 2015. Lecture.
- ↑ O'Hara, K.S., Walston, W.S., Ross, E.W., Darolia, R. US Patent 5482789, 1996.
- ↑ Chen, J.Y.; Feng, Q.; Sun, Z.Q. (October 2010). "Topologically close-packed phase promotion in a Ru-containing single crystal superalloy". Scripta Materialia. 63 (8): 795–798. doi:10.1016/j.scriptamat.2010.06.019.
- ↑ Wahl, Jacqueline, and Ken Harris. "New single crystal superalloys–overview and update." MATEC Web of Conferences. Vol. 14. EDP Sciences, 2014.
- ↑ Nabarro, F.R.N and de Villiers, H.L. "The physics of creep." Talylor and Francis, London, 1995
- ↑ N. Matan, D.C. Cox, P. Carter, M.A. Rist, C.M.F. Rae, R.C. Reed. "Creep of CMSX-4 superalloy single crystals: effects of misorientation and temperature."Acta Materialia. 47(1999)
- ↑ Reed RC. "The superalloys: fundamentals and applications." Cambridge: Cambridge Press; 2006.
- ↑ Frank R Nabarro. "Rafting in superalloys."1996
- ↑ R.C. Reed, N. Matan, D.C. Cox, M.A.Rist, C.M.F. Rae, acta Mater. 47(1999)
- 1 2 Pettit and Meier. "Oxidation and Hot Corrosion of Superalloys." TMS. 1984. 651-687
- ↑ Lund and Wagner. "Oxidation of Nickle- and Cobalt-Base Superalloys." DMIC report 214. 1965.
- ↑ Klein, L., Bauer, S., Neumeier, S., Göken, M., Virtanan, S. High temperature oxidation of γ/γ’-strengthened Co-based superalloys. Corrosion Science. 2011, 53:2027-2034.
- ↑ C. Sims, N. Stoloff, W. Hagel, Superalloys II: High Temperature Materials for Aerospace and Industrial Power, 1987, John Wiley & Sons
- ↑ IPMD. "PIM International Vol. 7 No. 1 March 2013". www.pim-international.com. Retrieved 2016-03-01.
- ↑ Atkinson, Dr H. V.; Davies, S. (2000-12-01). "Fundamental aspects of hot isostatic pressing: An overview". Metallurgical and Materials Transactions A. 31 (12): 2981–3000. doi:10.1007/s11661-000-0078-2. ISSN 1073-5623.
- ↑ Gu, D. D.; Meiners, W.; Wissenbach, K.; Poprawe, R. (2012-05-01). "Laser additive manufacturing of metallic components: materials, processes and mechanisms". International Materials Reviews. 57 (3): 133–164. doi:10.1179/1743280411Y.0000000014. ISSN 0950-6608.
- ↑ Y. Tamarin, Protective Coatings for Turbine Blades (Materials Park, OH: ASM International, 2002).
- ↑ J. R. Davis, ed., Handbook of Thermal Spray Technology (Materials Park, OH: The ASM Thermal Spray Society, 2004).
- ↑ D. H. Boone, Physical vapour deposition processes, Materials Science and Technology, 2 (1986), 220–224.
- ↑ D. R. Clarke, Materials selection guidelines for low thermal conductivity thermal barrier coatings, Surface and Coatings Technology, 163–164 (2003), 67–74.
- ↑ "Wadley Research Group - UVA". www.virginia.edu. Retrieved 2016-03-03.
- ↑ B. M. Warnes, Surf. Coat. Technol. 2003, 163–164, 106.
- ↑ H. M. Tawancy, N. M. Abbas, A. Bennett, Surf. Coat. Technol. 1994, 68–69, 10.
- ↑ C. Chuanxian, H. Bingtang, L. Huiling, Thin Solid Films 1984, 118, 485.
- ↑ Y. Kawahara, Mater. High Temp. 1997, 14, 261.
- ↑ Y. Longa-Nava, M. Takemoto, Corros. 1992, 48, 599.
- ↑ G. R. Heath, P. Heimgartner, G. Irons, R. Miller, S. Gustafsson, Mater. Sci. Forum 1997, 251–54, 809
- ↑ O. Knotek, Handbook of Hard Coatings: Deposition Technologies, Properties and Applications, Ed. R. F. Bunshah, Noyes Pub. Park Ridge, New Jersey, U. S. A./William Andrew Publishing, LLC, Norwich, New York, U.S.A. 2001.
- ↑ P. Niranatlumpong, C. B. Ponton, H. E. Evans, Oxid. Met. 2000, 53, 241
- ↑ P. Fauchais, A. Vardelle, M. Vardelle, Modelling of Plasma Spraying of Ceramic Films and Coatings, Ed. Vinenzini, Pub. Elsevier State Publishers B.V 1991.
- ↑ A. G. Evans, D. R. Mumm, J.W. Hutchinson, G. H. Meier and F. S. Pettit, Mechanisms controlling the durability of thermal barrier coatings, Progress in Materials Science, 46 (2001), 505–553.
- ↑ P. K. Wright and A. G. Evans, Mechanisms governing the performance of thermal barrier coatings, Current Opinion in Solid State and Materials Science, 4 (1999),255–265.
- ↑ P. K. Wright, Influence of cyclic strain on life of a PVD TBC, Materials Science and Engineering, A245 (1998), 191–200.
- ↑ B. A. Pint, The role of chemical composition on the oxidation performance of aluminide coatings, Surface and Coatings Technology, 188–189 (2004), 71–78.
- ↑ B. Baufeld, M. Bartsch, P. Broz and M. Schmucker, Microstructural changes as postmortem temperature indicator in Ni-Co-Cr-Al-Y oxidation protection coatings, Materials Science and Engineering, 384 (2004), 162–171.
- ↑ J. A. Nychka and D. R. Clarke, Damage quantification in TBCs by photo-stimulated luminescence spectroscopy, Surface and Coatings Technology, 146–147 (2001), 110–116.
- ↑ D. R. Mumm, A. G. Evans and I. T. Spitsberg, Characterisation of a cyclic displacement instability for a thermally grown oxide in a thermal barrier coating system, Acta Materialia, 49 (2001), 2329–2340.
- ↑ D. R. Mumm and A. G. Evans, On the role of imperfections in the failure of a thermal barrier coating made by electron beam deposition, Acta Materialia, 48(2000), 1815–1827.
- ↑ M. Gell, K. Vaidyanathan, B. Barber, J. Cheng and E. Jordan, Mechanism of spallation in platinum aluminide/electron beam physical vapor-deposited thermal barrier coatings, Metallurgical and Materials Transactions, 30A (1999), 427–435.
- ↑ A. G. Evans, M.Y. He and J.W. Hutchinson, Mechanics-based scaling laws for the durability of thermal barrier coatings, Progress in Materials Science, 46 (2001), 249–271.
- ↑ U. Schulz, M. Menzebach, C. Leyens and Y.Q. Yang, Influence of substrate material on oxidation behaviour and cyclic lifetime of EB-PVD TBC systems, Surface and Coatings Technology, 146–147 (2001), 117–123.
- ↑ X. Chen, R.Wang, N. Yao, A. G. Evans, J.W. Hutchinson and R.W. Bruce, Foreign object damage in a thermal barrier system: mechanisms and simulations, Materials Science and Engineering, A352 (2003), 221–231.
- ↑ W. S. Walston, Coating and surface technologies for turbine aerofoils, in K. A. Green, T. M. Pollock, H. Harada et al., Superalloys 2004 (Warrendale, PA: The Minerals, Metals and Materials Society (TMS), 2004), pp. 579–588.
- ↑ D. R. Mumm, M. Watanabe, A. G. Evans and J. A. Pfaendtner, The influence of test method on failure mechanisms and durability of a thermal barrier system, Acta Materialia, 52 (2004), 1123–1131.
- ↑ http://www.tms.org/meetings/specialty/superalloys2000/superalloyshistory.html
Bibliography
- Levitin, Valim (2006). High Temperature Strain of Metals and Alloys: Physical Fundamentals. WILEY-VCH. ISBN 978-3-527-31338-9.