Bipedalism

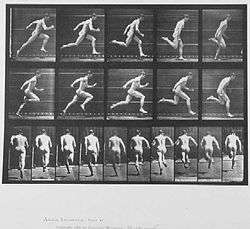
Bipedalism is a form of terrestrial locomotion where an organism moves by means of its two rear limbs or legs. An animal or machine that usually moves in a bipedal manner is known as a biped /ˈbaɪpɛd/, meaning "two feet" (from the Latin bis for "double" and pes for "foot"). Types of bipedal movement include walking, running, or hopping.
Few modern species are habitual bipeds whose normal method of locomotion is two-legged. Within mammals, habitual bipedalism has evolved multiple times, with the macropods, kangaroo rats and mice, springhare,[4] hopping mice, pangolins and homininan apes, as well as various other extinct groups evolving the trait independently. In the Triassic period some groups of archosaurs (a group that includes the ancestors of crocodiles) developed bipedalism; among their descendants the dinosaurs, all the early forms and many later groups were habitual or exclusive bipeds; the birds descended from one group of exclusively bipedal dinosaurs.
A larger number of modern species intermittently or briefly use a bipedal gait. Several non-archosaurian lizard species move bipedally when running, usually to escape from threats. Many primate and bear species will adopt a bipedal gait in order to reach food or explore their environment. Several arboreal primate species, such as gibbons and indriids, exclusively walk on two legs during the brief periods they spend on the ground. Many animals rear up on their hind legs whilst fighting or copulating. Some animals commonly stand on their hind legs, in order to reach food, to keep watch, to threaten a competitor or predator, or to pose in courtship, but do not move bipedally.
Definition
The word is derived from the Latin words bi(s) 'two' and ped- 'foot', as contrasted with quadruped 'four feet'.
Advantages
Limited and exclusive bipedalism can offer a species several advantages. Bipedalism raises the head; this allows a greater field of vision with improved detection of distant dangers or resources, access to deeper water for wading animals and allows the animals to reach higher food sources with their mouths. While upright, non-locomotory limbs become free for other uses, including manipulation (in primates and rodents), flight (in birds), digging (in giant pangolin), combat (in bears, great apes and the large monitor lizard) or camouflage (in certain species of octopus). The maximum bipedal speed appears less fast than the maximum speed of quadrupedal movement with a flexible backbone – both the ostrich and the red kangaroo can reach speeds of 70 km/h (43 mph), while the cheetah can exceed 100 km/h (62 mph).[5][6] Bipedality in kangaroo rats has been hypothesized to improve locomotor performance, which could aid in escaping from predators.[7][8]
Facultative and obligate bipedalism
Zoologists often label behaviors, including bipedalism, as "facultative" (i.e. optional) or "obligate" (the animal has no reasonable alternative). Even this distinction is not completely clear-cut — for example, humans other than infants normally walk and run in biped fashion, but almost all can crawl on hands and knees when necessary. There are even reports of humans who normally walk on all fours with their feet but not their knees on the ground, but these cases are a result of conditions such as Uner Tan syndrome — very rare genetic neurological disorders rather than normal behavior.[9] Even if one ignores exceptions caused by some kind of injury or illness, there are many unclear cases, including the fact that "normal" humans can crawl on hands and knees. This article therefore avoids the terms "facultative" and "obligate", and focuses on the range of styles of locomotion normally used by various groups of animals.
Movement
There are a number of states of movement commonly associated with bipedalism.
- Standing. Staying still on both legs. In most bipeds this is an active process, requiring constant adjustment of balance.
- Walking. One foot in front of another, with at least one foot on the ground at any time.
- Running. One foot in front of another, with periods where both feet are off the ground.
- Jumping/hopping. Moving by a series of jumps with both feet moving together.
Bipedal animals
The great majority of living terrestrial vertebrates are quadrupeds, with bipedalism exhibited by only a handful of living groups. Humans, gibbons and large birds walk by raising one foot at a time. On the other hand, most macropods, smaller birds, lemurs and bipedal rodents move by hopping on both legs simultaneously. Tree kangaroos are able to walk or hop, most commonly alternating feet when moving arboreally and hopping on both feet simultaneously when on the ground.
Amphibians
There are no known living or fossil bipedal amphibians.
Extant reptiles
Many species of lizards become bipedal during high-speed, sprint locomotion, including the world's fastest lizard, the spiny-tailed iguana (genus Ctenosaura).
Early reptiles and lizards
The first known biped is the bolosaurid Eudibamus whose fossils date from 290 million years ago.[10][11] Its long hindlegs, short forelegs, and distinctive joints all suggest bipedalism. The species was extinct before the dinosaurs appeared.
Archosaurs (include birds, crocodiles, and dinosaurs)
Birds
All birds are bipeds when on the ground, a feature inherited from their dinosaur ancestors.
Other archosaurs
Bipedalism evolved more than once in archosaurs, the group that includes both dinosaurs and crocodilians.[12] All dinosaurs are thought to be descended from a fully bipedal ancestor, perhaps similar to Eoraptor. Bipedal movement also re-evolved in a number of other dinosaur lineages such as the iguanodons. Some extinct members of the crocodilian line, a sister group to the dinosaurs and birds, also evolved bipedal forms - a crocodile relative from the triassic, Effigia okeeffeae, is thought to be bipedal.[13] Pterosaurs were previously thought to have been bipedal, but recent trackways have all shown quadrupedal locomotion. Bipedalism also evolved independently among the dinosaurs. Dinosaurs diverged from their archosaur ancestors approximately 230 million years ago during the Middle to Late Triassic period, roughly 20 million years after the Permian-Triassic extinction event wiped out an estimated 95% of all life on Earth.[14][15] Radiometric dating of fossils from the early dinosaur genus Eoraptor establishes its presence in the fossil record at this time. Paleontologists suspect Eoraptor resembles the common ancestor of all dinosaurs;[16] if this is true, its traits suggest that the first dinosaurs were small, bipedal predators.[17] The discovery of primitive, dinosaur-like ornithodirans such as Marasuchus and Lagerpeton in Argentinian Middle Triassic strata supports this view; analysis of recovered fossils suggests that these animals were indeed small, bipedal predators.
Mammals
A number of groups of extant mammals have independently evolved bipedalism as their main form of locomotion - for example humans, giant pangolins, the extinct giant ground sloths, numerous species of jumping rodents and macropods. Humans, as their bipedalism has been extensively studied, are documented in the next section. Macropods are believed to have evolved bipedal hopping only once in their evolution, at some time no later than 45 million years ago.[18] Bipedal movement is less common among mammals, most of which are quadrupedal. All primates possess some bipedal ability, though most species primarily use quadrupedal locomotion on land. Primates aside, the macropods (kangaroos, wallabies and their relatives), kangaroo rats and mice, hopping mice and springhare move bipedally by hopping. Very few mammals other than primates commonly move bipedally by an alternating gait rather than hopping. Exceptions are the ground pangolin and in some circumstances the tree kangaroo. One black bear "Pedals" became famous locally and on the internet for having a frequent bipedal gait, although this is attributed to injuries on the bear's front paws.
Primates
Most bipedal animals move with their backs close to horizontal, using a long tail to balance the weight of their bodies. The primate version of bipedalism is unusual because the back is close to upright (completely upright in humans). Many primates can stand upright on their hind legs without any support. Chimpanzees, bonobos, gibbons[19] and baboons[20] exhibit forms of bipedalism. Injured chimpanzees and bonobos have been capable of sustained bipedalism.[21] Geladas, although often quadrupedal, will move between adjacent feeding patches with a squatting, shuffling bipedal form of locomotion . Three captive primates, one macaque Natasha[22] and two chimps, Oliver and Poko (chimpanzee), were found to move bipedally . Natasha switched to exclusive bipedalism after an illness, while Poko was discovered in captivity in a tall, narrow cage.[23][24] Oliver reverted to knuckle-walking after developing arthritis. Non-human primates often use bipedal locomotion when carrying food.
The evolution of human bipedalism, began in primates about four million years ago,[25] or as early as seven million years ago with Sahelanthropus.[26] One hypothesis for human bipedalism is that it evolved as a result of differentially successful survival from carrying food to share with group members,[27] although there are other hypotheses, as discussed below.
Limited bipedalism
Limited bipedalism in mammals
Other mammals engage in limited, non-locomotory, bipedalism. A number of other animals, such as rats, raccoons, and beavers will squat on their hindlegs to manipulate some objects but revert to four limbs when moving (the beaver will move bipedally if transporting wood for their dams, as will the raccoon when holding food). Bears will fight in a bipedal stance to use their forelegs as weapons. A number of mammals will adopt a bipedal stance in specific situations such as for feeding or fighting. Ground squirrels and meerkats will stand on hind legs to survey their surroundings, but will not walk bipedally. Dogs (e.g. Faith) can stand or move on two legs if trained, or if birth defect or injury precludes quadrupedalism. The gerenuk antelope stands on its hind legs while eating from trees, as did the extinct giant ground sloth and chalicotheres. The spotted skunk will walk on its front legs when threatened, rearing up on its front legs while facing the attacker so that its anal glands, capable of spraying an offensive oil, face its attacker.
Limited bipedalism in non-mammals
Bipedalism is unknown among the amphibians. Among the non-archosaur reptiles bipedalism is rare, but it is found in the 'reared-up' running of lizards such as agamids and monitor lizards. Many reptile species will also temporarily adopt bipedalism while fighting.[28] One genus of basilisk lizard can run bipedally across the surface of water for some distance. Among arthropods, cockroaches are known to move bipedally at high speeds.[29] Bipedalism is rarely found outside terrestrial animals, though at least two types of octopus walk bipedally on the sea floor using two of their arms, allowing the remaining arms to be used to camouflage the octopus as a mat of algae or a floating coconut.[30]
Evolution of human bipedalism
There are at least twelve distinct hypotheses as to how and why bipedalism evolved in humans, and also some debate as to when. Bipedalism evolved well before the large human brain or the development of stone tools.[31] Bipedal specializations are found in Australopithecus fossils from 4.2-3.9 million years ago,[32] although Sahelanthropus may have walked on two legs as early as seven million years ago.[26] Nonetheless, the evolution of bipedalism was accompanied by significant evolutions in the spine including the forward movement in position of the foramen magnum, where the spinal cord leaves the cranium.[33] Recent evidence regarding modern human sexual dimorphism (physical differences between male and female) in the lumbar spine has been seen in pre-modern primates such as Australopithecus africanus. This dimorphism has been seen as an evolutionary adaptation of females to bear lumbar load better during pregnancy, an adaptation that non-bipedal primates would not need to make.[34][35] Adapting bipedalism would have required less shoulder stability, which allowed the shoulder and other limbs to become more independent of each other and adapt for specific suspensory behaviors. In addition to the change in shoulder stability, changing locomotion would have increased the demand for shoulder mobility, which would have propelled the evolution of bipedalism forward.[36] The different hypotheses are not necessarily mutually exclusive and a number of selective forces may have acted together to lead to human bipedalism. It is important to distinguish between adaptations for bipedalism and adaptations for running, which came later still.
Possible reasons for the evolution of human bipedalism include freeing the hands for tool use and carrying, sexual dimorphism in food gathering, changes in climate and habitat (from jungle to savanna) that favored a more elevated eye-position, and to reduce the amount of skin exposed to the tropical sun. It is possible that bipedalism provided a variety of benefits to the hominin species, and scientists have suggested multiple reasons for evolution of human bipedalism.[37] There also is not only question of why were the earliest hominins partially bipedal but also why did hominins become more bipedal over time. For example, the postural feeding hypothesis (reaching for food/balancing) provides an explanation for how earliest hominins became for the benefit of reaching out for food in trees while the savannah-based theory describes how the late hominins that started to settle on the ground became increasingly bipedal.[38]
Multiple Factors
Napier (1963) argued that it was very unlikely that single factor drove the evolution of Bipedalism. He stated "It seems unlikely that any single factor was responsible for such a dramatic change in behaviour. In addition to the advantages of accruing from ability to carry objects - food or otherwise - the improvement of the visual range and the freeing of the hands for purposes of defence and offence must equally have played their part as catalysts.” [39] Sigmon argued that chimpanzees demonstrate bipedalism in different contexts, and one single factor should be used to explain bipedalism. preadaptation for human bipedalism.[40] Day (1986) emphasized three major pressures that drove evolution of bipedalism 1.food acquisition 2. predator avoidance 3. Reproductive success.[41] Ko (2015) states there are two questions regarding bipedalism 1. Why were the earliest hominins partially bipedal 2. why did hominins become more bipedal over time. He argues that these questions can be answered with combination of prominent theories such as Savanna-based, Postural feeding, and Provisioning.[42]
Savanna-based theory
According to the savanna-based theory, hominines descended from the trees and adapted to life on the savanna by walking erect on two feet. The theory suggests that early hominids were forced to adapt to bipedal locomotion on the open savanna after they left the trees. This theory is closely related to the knuckle-walking hypothesis, which states that human ancestors used quadrupedal locomotion on the savanna, as evidenced by morphological characteristics found in Australopithecus anamensis and Australopithecus afarensis forelimbs, and that it is less parsimonious to assume that knuckle walking developed twice in genera Pan and Gorilla instead of evolving it once as synapomorphy for Pan and Gorilla before losing it in Australopithecus.[43] The evolution of an orthograde posture would have been very helpful on a savanna as it would allow the ability to look over tall grasses in order to watch out for predators, or terrestrially hunt and sneak up on prey.[44] It was also suggested in P.E. Wheeler's "The evolution of bipedality and loss of functional body hair in hominids", that a possible advantage of bipedalism in the savanna was reducing the amount of surface area of the body exposed to the sun, helping regulate body temperature.[45] In fact, Elizabeth Vrba’s turnover pulse hypothesis supports the savanna-based theory by explaining the shrinking of forested areas due to global warming and cooling, which forced animals out into the open grasslands and caused the need for hominids to acquire bipedality.[46]
Rather, the bipedal adaptation hominines had already achieved was used in the savanna. The fossil record shows that early bipedal hominines were still adapted to climbing trees at the time they were also walking upright. It is possible that Bipedalism evolved in the trees, and was later applied to the Savannah as a vestigial trait. Humans and orangutans are both unique to a bipedal reactive adaptation when climbing on thin branches, in which they have increased hip and knee extension in relation to the diameter of the branch, which can increase an arboreal feeding range and can be attributed to a convergent evolution of bipedalism evolving in arboreal environments.[47] Hominine fossils found in dry grassland environments led anthropologists to believe hominines lived, slept, walked upright, and died only in those environments because no hominine fossils were found in forested areas. However, fossilization is a rare occurrence—the conditions must be just right in order for an organism that dies to become fossilized for somebody to find later, which is also a rare occurrence. The fact that no hominine fossils were found in forests does not ultimately lead to the conclusion that no hominines ever died there. The convenience of the savanna-based theory caused this point to be overlooked for over a hundred years.[48]
Some of the fossils found actually showed that there was still an adaptation to arboreal life. For example, Lucy, the famous Australopithecus afarensis, found in Hadar in Ethiopia, which may have been forested at the time of Lucy’s death, had curved fingers that would still give her the ability to grasp tree branches, but she walked bipedally. “Little Foot,” the collection of Australopithecus africanus foot bones, has a divergent big toe as well as the ankle strength to walk upright. “Little Foot” could grasp things using his feet like an ape, perhaps tree branches, and he was bipedal. Ancient pollen found in the soil in the locations in which these fossils were found suggest that the area used to be much more wet and covered in thick vegetation and has only recently become the arid desert it is now.[46]
Traveling efficiency hypothesis
An alternative explanation is the mixture of savanna and scattered forests increased terrestrial travel by proto-humans between clusters of trees, and bipedalism offered greater efficiency for long-distance travel between these clusters than quadrupedalism.[49][50] In an experiment monitoring chimpanzee metabolic rate via oxygen consumption, it was found that the quadrupedal and bipedal energy costs were very similar, implying that this transition in early ape-like ancestors would have not have been very difficult or energetically costing.[51] This increased travel efficiency is likely to have been selected for as it assisted the wide dispersal of early hominids across the Savannah to create start populations.
Postural feeding hypothesis
The postural feeding hypothesis has been recently supported by Dr. Kevin Hunt, a professor at Indiana University. This hypothesis asserts that chimpanzees were only bipedal when they eat. While on the ground, they would reach up for fruit hanging from small trees and while in trees, bipedalism was used to reach up to grab for an overhead branch. These bipedal movements may have evolved into regular habits because they were so convenient in obtaining food. Also, Hunt's hypotheses states that these movements coevolved with chimpanzee arm-hanging, as this movement was very effective and efficient in harvesting food. When analyzing fossil anatomy, Australopithecus afarensis has very similar features of the hand and shoulder to the chimpanzee, which indicates hanging arms. Also, the Australopithecus hip and hind limb very clearly indicate bipedalism, but these fossils also indicate very inefficient locomotive movement when compared to humans. For this reason, Hunt argues that bipedalism evolved more as a terrestrial feeding posture than as a walking posture.
A similar study conducted by Thorpe et al. looked at how the most arboreal great ape, the orangutan, held onto supporting branches in order to navigate branches that were too flexible or unstable otherwise. They found that in more than 75% of locomotive instances the orangutans used their hands to stabilize themselves while they navigated thinner branches. They hypothesized that increased fragmentation of forests where A. afarensis as well as other ancestors of modern humans and other apes resided could have contributed to this increase of bipedalism in order to navigate the diminishing forests. Their findings also shed light on a couple of discrepancies observed in the anatomy of A. afarensis, such as the ankle joint, which allowed it to “wobble” and long, highly flexible forelimbs. The idea that bipedalism started from walking in trees explains both the increased flexibility in the ankle as well as the long limbs which would be used to grab hold of branches.
Provisioning model
One theory on the origin of bipedalism is the behavioral model presented by C. Owen Lovejoy, known as "male provisioning".[52] Lovejoy theorizes that the evolution of bipedalism was linked to monogamy. In the face of long inter-birth intervals and low reproductive rates typical of the apes, early hominids engaged in pair-bonding that enabled greater parental effort directed towards rearing offspring. Lovejoy proposes that male provisioning of food would improve the offspring survivorship and increase the pair's reproductive rate. Thus the male would leave his mate and offspring to search for food and return carrying the food in his arms walking on his legs. This model is supported by the reduction ("feminization") of the male canine teeth in early hominids such as Sahelanthropus tchadensis[53] and Ardipithecus ramidus,[54] which along with low body size dimorphism in Ardipithecus[55] and Australopithecus,[56] suggests a reduction in inter-male antagonistic behavior in early hominids.[57] In addition, this model is supported by a number of modern human traits associated with concealed ovulation (permanently enlarged breasts, lack of sexual swelling) and low sperm competition (moderate sized testes, low sperm mid-piece volume) that argues against recent adaptation to a polygynous reproductive system.[57]
However, this model has generated some controversy, as others have argued that early bipedal hominids were instead polygynous. Among most monogamous primates, males and females are about the same size. That is sexual dimorphism is minimal, and other studies have suggested that Australopithecus afarensis males were nearly twice the weight of females. However, Lovejoy's model posits that the larger range a provisioning male would have to cover (to avoid competing with the female for resources she could attain herself) would select for increased male body size to limit predation risk.[58] Furthermore, as the species became more bipedal, specialized feet would prevent the infant from conveniently clinging to the mother - hampering the mother's freedom[59] and thus make her and her offspring more dependent on resources collected by others. Modern monogamous primates such as gibbons tend to be also territorial, but fossil evidence indicates that Australopithecus afarensis lived in large groups. However, while both gibbons and hominids have reduced canine sexual dimorphism, female gibbons enlarge ('masculinize') their canines so they can actively share in the defense of their home territory. Instead, the reduction of the male hominid canine is consistent with reduced inter-male aggression in a group living primate.
Early bipedalism in homininae model
Recent studies of 4.4 million years old Ardipithecus ramidus suggest bipedalism, it is thus possible that bipedalism evolved very early in homininae and was reduced in chimpanzee and gorilla when they became more specialized. According to Richard Dawkins in his book "The Ancestor's Tale", chimps and bonobos are descended from Australopithecus gracile type species while gorillas are descended from Paranthropus. These apes may have once been bipedal, but then lost this ability when they were forced back into an arboreal habitat, presumably by those australopithecines who eventually became us (see Homininae). Early homininaes such as Ardipithecus ramidus may have possessed an arboreal type of bipedalism that later independently evolved towards knuckle-walking in chimpanzees and gorillas[60] and towards efficient walking and running in modern humans (see figure). It is also proposed that one cause of Neanderthal extinction was a less efficient running.
Warning display (aposematic) model
Joseph Jordania from the University of Melbourne recently (2011) suggested that bipedalism was one of the central elements of the general defense strategy of early hominids, based on aposematism, or warning display and intimidation of potential predators and competitors with exaggerated visual and audio signals. According to this model, hominids were trying to stay as visible and as loud as possible all the time. Several morphological and behavioral developments were employed to achieve this goal: upright bipedal posture, longer legs, long tightly coiled hair on the top of the head, body painting, threatening synchronous body movements, loud voice and extremely loud rhythmic singing/stomping/drumming on external subjects.[61] Slow locomotion and strong body odor (both characteristic for hominids and humans) are other features often employed by aposematic species to advertise their non-profitability for potential predators.
Other behavioural models
There are a variety of ideas which promote a specific change in behaviour as the key driver for the evolution of hominid bipedalism. For example, Wescott (1967) and later Jablonski & Chaplin (1993) suggest that bipedal threat displays could have been the transitional behaviour which led to some groups of apes beginning to adopt bipedal postures more often. Others (e.g. Dart 1925) have offered the idea that the need for more vigilance against predators could have provided the initial motivation. Dawkins (e.g. 2004) has argued that it could have begun as a kind of fashion that just caught on and then escalated through sexual selection. And it has even been suggested (e.g. Tanner 1981:165) that male phallic display could have been the initial incentive, as well as increased sexual signaling in upright female posture.[44]
Thermoregulatory model
The thermoregulatory model explaining the origin of bipedalism is one of the simplest theories so far advanced, but it is a viable explanation. Dr. Peter Wheeler, a professor of evolutionary biology, proposes that bipedalism raises the amount of body surface area higher above the ground which results in a reduction in heat gain and helps heat dissipation.[62][63][64] When a hominid is higher above the ground, the organism accesses more favorable wind speeds and temperatures. During heat seasons, greater wind flow results in a higher heat loss, which makes the organism more comfortable. Also, Wheeler explains that a vertical posture minimizes the direct exposure to the sun whereas quadrupedalism exposes more of the body to direct exposure. Analysis and interpretations of Ardipithecus reveal that this hypothesis needs modification to consider that the forest and woodland environmental preadaptation of early-stage hominid bipedalism preceded further refinement of bipedalism by the pressure of natural selection. This then allowed for the more efficient exploitation of the hotter conditions ecological niche, rather than the hotter conditions being hypothetically bipedalism's initial stimulus. A feedback mechanism from the advantages of bipedality in hot and open habitats would then in turn make a forest preadaptation solidify as a permanent state.[65]
Carrying models
Charles Darwin wrote that "Man could not have attained his present dominant position in the world without the use of his hands, which are so admirably adapted to the act of obedience of his will" Darwin (1871:52) and many models on bipedal origins are based on this line of thought. Gordon Hewes (1961) suggested that the carrying of meat "over considerable distances" (Hewes 1961:689) was the key factor. Isaac (1978) and Sinclair et al. (1986) offered modifications of this idea as indeed did Lovejoy (1981) with his 'provisioning model' described above. Others, such as Nancy Tanner (1981) have suggested that infant carrying was key, whilst others have suggested stone tools and weapons drove the change.[66] This stone tools theory is very unlikely, as though ancient humans were known to hunt, the discovery of tools was not discovered for thousands of years after the origin of bipedalism, temporally preventing it from being a driving force of evolution. (Wooden tools and spears fossilize poorly and therefore it's difficult to make a judgement about their potential usage.)
Wading models
The observation that large Primates, including especially the great apes, that predominantly move quadrupedally on dry land, tend to switch to bipedal locomotion in waist deep water, has led to the idea that the origin of human bipedalism may have been influenced by waterside environments. This idea, labelled "The Wading Hypothesis",[67] was originally promoted by Elaine Morgan, as part of the aquatic ape hypothesis, who cited bipedalism among a cluster of other human traits unique among primates, including voluntary control of breathing, hairlessness and subcutaneous fat. She argued that wading, swimming and diving through water offer better explanations for these traits than more conventional theories.[68] The "aquatic ape hypothesis", as originally formulated, has not been accepted or considered a serious theory within the anthropological scholarly community.[69] Others, however, have sought to promote wading as a factor in the origin of human bipedalism without referring to further ("aquatic ape" related) factors. Since 2000 Carsten Niemitz has published a series of papers and a book [70] on a variant of the wading hypothesis, which he calls The Amphibian Generalist Theory. ("Amphibische Generalistentheorie").
Other theories have been proposed that suggest wading and the exploitation of aquatic food sources (providing essential nutrients for human brain evolution[71] or critical fallback foods[72]) may have exerted evolutionary pressures on human ancestors promoting adaptations which later assisted full-time bipedalism. It has also been thought that consistent water-based food sources had developed early hominid dependency and facilitated dispersal along seas and rivers.[73]
Physiology
Bipedal movement occurs in a number of ways, and requires many mechanical and neurological adaptations. Some of these are described below.
Biomechanics
Standing
Energy-efficient means of standing bipedally involve constant adjustment of balance, and of course these must avoid overcorrection. The difficulties associated with simple standing in upright humans are highlighted by the greatly increased risk of falling present in the elderly, even with minimal reductions in control system effectiveness.
Shoulder stability
Shoulder stability would decrease with the evolution of bipedalism. Shoulder mobility would increase because the need for a stable shoulder is only present in arboreal habitats. Shoulder mobility would support suspensory locomotion behaviors which are present in human bipedalism. The forelimbs are freed from weight bearing capabilities which makes the shoulder a place of evidence for the evolution of bipedalism.[74]
Walking
Walking is characterized by an "inverted pendulum" movement in which the center of gravity vaults over a stiff leg with each step.[75] Force plates can be used to quantify the whole-body kinetic & potential energy, with walking displaying an out-of-phase relationship indicating exchange between the two.[75] Interestingly, this model applies to all walking organisms regardless of the number of legs, and thus bipedal locomotion does not differ in terms of whole-body kinetics.[76]
In humans, walking is composed of several separate processes:[75]
- Vaulting over a stiff stance leg
- Passive ballistic movement of the swing leg
- A short 'push' from the ankle prior to toe-off, propelling the swing leg
- Rotation of the hips about the axis of the spine, to increase stride length
- Rotation of the hips about the horizontal axis to improve balance during stance
Running
Running is characterized by a spring-mass movement.[75] Kinetic and potential energy are in phase, and the energy is stored & released from a spring-like limb during foot contact.[75] Again, the whole-body kinetics are similar to animals with more limbs.[76]
Musculature
Bipedalism requires strong leg muscles, particularly in the thighs. Contrast in domesticated poultry the well muscled legs, against the small and bony wings. Likewise in humans, the quadriceps and hamstring muscles of the thigh are both so crucial to bipedal activities that each alone is much larger than the well-developed biceps of the arms.
Respiration
A biped has the ability to breathe while running, without strong coupling to stride cycle. Humans usually take a breath every other stride when their aerobic system is functioning. During a sprint the anaerobic system kicks in and breathing slows until the anaerobic system can no longer sustain a sprint.
Bipedal robots

For nearly the whole of the 20th century, bipedal robots were very difficult to construct and robot locomotion involved only wheels, treads, or multiple legs. Recent cheap and compact computing power has made two-legged robots more feasible. Some notable biped robots are ASIMO, HUBO, MABEL and QRIO. Recently, spurred by the success of creating a fully passive, un-powered bipedal walking robot,[77] those working on such machines have begun using principles gleaned from the study of human and animal locomotion, which often relies on passive mechanisms to minimize power consumption.
See also
Notes
- ↑ The red kangaroo can attain a similar speed for short distances.[3]
References
- ↑ Stewart, D. (2006-08-01). "A Bird Like No Other". National Wildlife. National Wildlife Federation. Archived from the original on 2012-02-09. Retrieved 2014-05-30.
- ↑ Davies, S.J.J.F. (2003). "Birds I Tinamous and Ratites to Hoatzins". In Hutchins, Michael. Grzimek's Animal Life Encyclopedia. 8 (2 ed.). Farmington Hills, MI: Gale Group. pp. 99–101. ISBN 0-7876-5784-0.
- ↑ Penny, M. (2002). The Secret World of Kangaroos. Austin TX: Raintree Steck-Vaughn. ISBN 0-7398-4986-7.
- ↑ Heglund, NC; Cavagna, GA; Taylor, CR (1982). "Energetics and mechanics of terrestrial locomotion. III. Energy changes of the centre of mass as a function of speed and body size in birds and mammals". Journal of Experimental Biology. 97.
- ↑ Garland, T. Jr. (1983). "The relation between maximal running speed and body mass in terrestrial mammals" (PDF). Journal of Zoology, London. 199 (2): 157–170. doi:10.1111/j.1469-7998.1983.tb02087.x.
- ↑ Sharp, N.C.C. (1997). "Timed running speed of a cheetah (Acinonyx jubatus)". Journal of Zoology. 241 (3): 493–494. doi:10.1111/j.1469-7998.1997.tb04840.x.
- ↑ Djawdan, M (1993). "Locomotor performance of bipedal and quadrupedal heteromyid rodents". Functional Ecology. British Ecological Society. 7 (2): 195–202. doi:10.2307/2389887. JSTOR 2389887.
- ↑ Djawdan, M.; Garland, T. Jr. (1988). "Maximal running speeds of bipedal and quadrupedal rodents" (PDF). Journal of Mammalogy. American Society of Mammalogists. 69 (4): 765–772. doi:10.2307/1381631. JSTOR 1381631.
- ↑ Humphrey, N.; Skoyles, J.R.; Keynes, R. (2005). "Human Hand-Walkers: Five Siblings Who Never Stood Up" (PDF). Centre for Philosophy of Natural and Social Science, London School of Economics.
- ↑ "Upright lizard leaves dinosaur standing". cnn.com. 2000-11-03. Archived from the original on 2007-10-31. Retrieved 2007-10-17.
- ↑ Berman, David S.; et al. (2000). "Early Permian Bipedal Reptile". Science. 290 (5493): 969–972. Bibcode:2000Sci...290..969B. doi:10.1126/science.290.5493.969. PMID 11062126.
- ↑ Hutchinson, J.R. (2006). "The evolution of locomotion in archosaurs". Comptes Rendus Palevol. 5 (3–4): 519–530. doi:10.1016/j.crpv.2005.09.002.
- ↑ Handwerk, Brian (2006-01-26). "Dino-Era Fossil Reveals Two-Footed Croc Relative". National Geographic. Retrieved 2007-10-29.
- ↑ Citation for Permian/Triassic extinction event, percentage of animal species that went extinct. See commentary
- ↑ Another citation for P/T event data. See commentary
- ↑ Hayward, T. (1997). The First Dinosaurs. Dinosaur Cards. Orbis Publishing Ltd. D36040612.
- ↑ Sereno, Paul C.; Catherine A. Forster; Raymond R. Rogers; Alfredo M. Monetta (January 1993). "Primitive dinosaur skeleton from Argentina and the early evolution of Dinosauria". Nature. 361 (6407): 64–66. Bibcode:1993Natur.361...64S. doi:10.1038/361064a0. Retrieved 2008-06-28.
- ↑ Burk, Angela; Michael Westerman; Mark Springer (September 1988). "The Phylogenetic Position of the Musky Rat-Kangaroo and the Evolution of Bipedal Hopping in Kangaroos (Macropodidae: Diprotodontia)". Systematic Biology. 47 (3): 457–474. doi:10.1080/106351598260824. PMID 12066687.
- ↑ Aerts, Peter; Evie E. Vereeckea; Kristiaan D'Aoûta (2006). "Locomotor versatility in the white-handed gibbon (Hylobates lar): A spatiotemporal analysis of the bipedal, tripedal, and quadrupedal gaits". Journal of Human Evolution. Elsevier. 50 (5): 552–567. doi:10.1016/j.jhevol.2005.12.011. PMID 16516949.
- ↑ Rose, M.D. (1976). "Bipedal behavior of olive baboons (Papio anubis) and its relevance to an understanding of the evolution of human bipedalism". American Journal of Physical Anthropology. 44 (2): 247–261. doi:10.1002/ajpa.1330440207. PMID 816205.
- ↑ Bauer, Harold (1976). "Chimpanzee bipedal locomotion in the Gombe National Park, East Africa". Primates. 18 (4): 913–921. doi:10.1007/BF02382940.
- ↑ Waldman, Dan (2004-07-21). "Monkey apes humans by walking on two legs". MSNBC. Retrieved 2007-10-29.
- ↑ "University of Liverpool - Research Intelligence Issue 22 - Walking tall after all". Liv.ac.uk. Retrieved 2013-04-30.
- ↑ Tetrapod Zoology : Bipedal orangs, gait of a dinosaur, and new-look Ichthyostega: exciting times in functional anatomy part I Archived May 8, 2012, at the Wayback Machine.
- ↑ Kondō, Shirō (1985). Primate morphophysiology, locomotor analyses, and human bipedalism. Tokyo: University of Tokyo Press. ISBN 4-13-066093-4.
- 1 2 Staff (August 14, 2016). "What Does It Mean To Be Human? - Walking Upright". Smithsonian Institution. Retrieved August 14, 2016.
- ↑ "Bipedality in chimpanzee (Pan troglodytes) and bonobo (Pan paniscus): Testing hypotheses on the evolution of bipedalism". .interscience.wiley.com. 2002-05-09. Retrieved 2013-04-30.
- ↑ Sharma, Jayanth (2007-03-08). "The Story behind the Picture - Monitor Lizards Combat" (php). Wildlife Times. Retrieved 2007-10-29.
- ↑ "Bipedal animals, and their differences from humans". Ingentaconnect.com. 2004-05-01. Retrieved 2013-04-30.
- ↑ Huffard CL, Boneka F, Full RJ (2005). "Underwater bipedal locomotion by octopuses in disguise". Science. 307 (5717): 1927. doi:10.1126/science.1109616. PMID 15790846.
- ↑ Lovejoy, C.O. (1988). "Evolution of Human walking". Scientific American. 259 (5): 82–89. doi:10.1038/scientificamerican1188-118. PMID 3212438.
- ↑ McHenry, H.M (2009). "Human Evolution". In Michael Ruse & Joseph Travis. Evolution: The First Four Billion Years. Cambridge, Massachusetts: The Belknap Press of Harvard University Press. p. 263. ISBN 978-0-674-03175-3.
- ↑ Erin Wayman (August 6, 2012). "Becoming Human: The Evolution of Walking Upright". smithsonian.com.
- ↑ The Independent's article A pregnant woman's spine is her flexible friend, by Steve Connor from The Independent (Published: 13 December 2007) quoting Shapiro, Liza, University of Texas at Austin Dept. of Anthropology about her article, Whitcome, et al., Nature advance online publication, (2007). doi:10.1038/nature06342
- ↑ Why Pregnant Women Don't Tip Over. Amitabh Avasthi for National Geographic News, December 12, 2007. This article has good pictures explaining the differences between bipedal and non-bipedal pregnancy loads.
- ↑ Sylvester, Adam D. (2006). "Locomotor Coupling and the Origin of Hominin Bipedalism". Journal of Theoretical Biology. 242: 581–590. doi:10.1016/j.jtbi.2006.04.016.
- ↑ Sigmon, Becky (1971). "Bipedal behavior and the emergence of erect posture in man.". American Journal of Physical Anthropology. 34: 55–60. doi:10.1002/ajpa.1330340105.
- ↑ Ko, Kwang Hyun (2015). "Origins of Bipedalism". Brazilian Archives of Biology and Technology. 58: 929–934. doi:10.1590/S1516-89132015060399.
- ↑ Napier,, JR (1964). The evolution of bipedal walking in the hominids. Archives de Biologie (Liege).
- ↑ Sigmon, Becky (1971). "Bipedal behavior and the emergence of erect posture in man". American Journal of Physical Anthropology. 58: 929–934. doi:10.1590/S1516-89132015060399.
- ↑ Day, MH (1986). Bipedalism: Pressures, origins and modes. Major topics in human evolution. Cambridge: Cambridge University Press.
- ↑ Kwang Hyun, Ko (2015). "Origins of Bipedalism". Brazilian Archives of Biology and Technology. 58: 929–934. doi:10.1590/S1516-89132015060399.
- ↑ Richmond, B. G., and D. S. Strait. 2000. Evidence that humans evolved from a knuckle-walking ancestor. Nature: 382.
- 1 2 Dean, F. 2000. Primate diversity. W.W. Norton & Company, Inc: New York. Print.
- ↑ Wheeler, P. E., "The Evolution of Bipedality and Loss of Functional Body Hair in Hominoids." Journal of Human Evolution, 13, 91-98, (1984).
- 1 2 Shreeve, James, "Sunset on the savanna", Discover, 1996
- ↑ Thorpe S. K., R.L Holder, R. H. Crompton. 2007. Origin of human bipedalism as an adaptation for locomotion on flexible branches. Science: 1328-31.
- ↑ Shreeve, James, "Sunset on the savanna", ‘’Discover, 1996
- ↑ Isbell, L.A. & T.P. Young. (1996). "The evolution of bipedalism in hominids and reduced group size in chimpanzees: alternative responses to decreasing resource availability". Journal of Human Evolution. 30: 389–397. doi:10.1006/jhev.1996.0034.
- ↑ Lewin, Roger; Swisher, Carl Celso; Curtis, Garniss H. (2000). Java man: how two geologists' dramatic discoveries changed our understanding of the evolutionary path to modern humans. New York: Scribner. ISBN 0-684-80000-4.
- ↑ Pontzer, H.; Raichlen, D.A.; Rodman, P.S. (2014). "Bipedal and quadrupedal locomotion in chimpanzees". Journal of Human Evolution. 66: 64–82. doi:10.1016/j.jhevol.2013.10.002.
- ↑ T. Douglas Price; Gary M. Feinman (2003). Images of the Past, 5th edition. Boston: McGraw Hill. p. 68. ISBN 978-0-07-340520-9.
- ↑ Brunet, Michel; Guy F; Pilbeam D; Mackaye HT; Likius A; et al. (11 July 2002). "A new hominid from the Upper Miocene of Chad, Central Africa". Nature. 418 (6894): 145–151. doi:10.1038/nature00879. PMID 12110880.
- ↑ Suwa, Gen; Kono RT; Simpson SW; Asfaw B; Lovejoy CO; White TD (2 October 2009). "Paleobiological implications of the Ardipithecus ramidus dentition". Science. 326 (5949): 94–99. Bibcode:2009Sci...326...94S. doi:10.1126/science.1175824. PMID 19810195.
- ↑ White TD et al. Science. 2009 326(5949):75-86
- ↑ Reno PL et al. Philos Trans R Soc Lond B Biol Sci. 2010 365(1556):3355-63; Harmon E. J Hum Evol. 2009 56(6):551-9; Reno PL and Lovejoy CO. PeerJ. 2015. 3:e925 https://doi.org/10.7717/peerj.925
- 1 2 Lovejoy CO. Science. 2009 326(5949):74e1-8.
- ↑ Lovejoy CO. Science. 1981 211(4480):341-50.
- ↑ Keith Oatley, Dacher Keltner, Jennifer M. Jenkins. Understanding Emotion (2006) Second Edition. Page 235.
- ↑ Kivell TL, Schmitt D (August 2009). "Independent evolution of knuckle-walking in African apes shows that humans did not evolve from a knuckle-walking ancestor". Proc. Natl. Acad. Sci. U.S.A. 106 (34): 14241–6. Bibcode:2009PNAS..10614241K. doi:10.1073/pnas.0901280106. PMC 2732797
 . PMID 19667206.
. PMID 19667206. - ↑ Joseph Jordania. Why do People Sing? Music in Human Evolution. Logos, 2011
- ↑ Wheeler, P.E., 1984. The evolution of bipedality and loss of functional body hair in hominids. J. Hum. Evol. 13, 91e98.
- ↑ Wheeler, P.E., 1990. The influence of thermoregulatory selection pressures on hominid evolution. Behav. Brain. Sci. 13, 366e366.
- ↑ Wheeler, P.E., 1991. The influence of bipedalism on the energy and water budgets of early hominids. J. Hum. Evol. 21, 117e136.
- ↑ David-Barrett, T.; Dunbar, R. (2016). "Bipedality and hair loss in human evolution revisited: The impact of altitude and activity scheduling". J. Hum. Evol. 94: 72–82. doi:10.1016/j.jhevol.2016.02.006.
- ↑ Tanner, Nancy Makepeace " On Becoming Human" Cambridge: Cambridge University Press, 1981
- ↑ Kuliukas A (2013). "Wading Hypotheses of the Origin of Human Bipedalism". Human Evolution. 28 (3-4): 213–236.
- ↑ Morgan, Elaine (1997). The Aquatic Ape Hypothesis. Souvenir Press. ISBN 0-285-63518-2.
- ↑ Meier, R (2003). The complete idiot's guide to human prehistory. Alpha Books. pp. 57–59. ISBN 0-02-864421-2.
- ↑ Niemitz, Carsten (2004). Das Geheimnis des Aufrechten Gangs ~ Unsere Evolution Verlief Anders. Beck. ISBN 3-406-51606-8.
- ↑ Cunnane, Stephen C (2005). Survival of the fattest: the key to human brain evolution. World Scientific Publishing Company. pp. 259. ISBN 981-256-191-9.
- ↑ Wrangham R, Cheney D, Seyfarth R, Sarmiento E (December 2009). "Shallow-water habitats as sources of fallback foods for hominins". Am. J. Phys. Anthropol. 140 (4): 630–42. doi:10.1002/ajpa.21122. PMID 19890871.
- ↑ {Verhaegena, M., P. F. Puechb, S. Munro. 2002. Aquaboreal ancestors? Trends in Evolution and Ecology: 212 – 217.}
- ↑ Sylvester, Adam D (2006). "Locomotor Coupling and the Origin of Hominin Bipedalism". Journal of Theoretical Biology. 242: 581–590. doi:10.1016/j.jtbi.2006.04.016.
- 1 2 3 4 5 McMahon, Thomas A (1984). "Muscles, reflexes, and locomotion". ISBN 978-0-691-02376-2.
- 1 2 Biewener, Andrew A; Daniel, T (2003). "A moving topic: control and dynamics of animal locomotion". Biology Letters. 6 (3): 387–8. doi:10.1098/rsbl.2010.0294. ISBN 978-0-19-850022-3. PMC 2880073
 . PMID 20410030.
. PMID 20410030. - ↑ "Passive Dynamic Walking at Cornell". Ruina.tam.cornell.edu. Retrieved 2013-04-30.
Further reading
- Darwin, C., "The Descent of Man and Selection in Relation to Sex", Murray (London), (1871).
- Dart, R.A., "Australopithecus africanus: The Ape Man of South Africa" Nature, 145, 195-199, (1925).
- Dawkins, R., "The Ancestor's Tale", Weidenfeld and Nicolson (London), (2004).
- Hewes, G.W., "Food Transport and the Origin of Hominid Bipedalism" American Anthropologist, 63, 687-710, (1961).
- Hunt, K.D., "The Evolution of Human Bipedality" Journal of Human Evolution, 26, 183-202, (1994).
- Isaac, G.I., "The Archeological Evidence for the Activities of Early African Hominids" In:Early Hominids of Africa (Jolly, C.J. (Ed.)), Duckworth (London), 219-254, (1978).
- Jablonski, N.G.; Chaplin, G. (1993). "Origin of Habitual Terrestrial Bipedalism in the Ancestor of the Hominidae". Journal of Human Evolution. 24: 259–280. doi:10.1006/jhev.1993.1021.
- Lovejoy, C. O. (1981). "The Origin of Man". Science. 211 (4480): 341–350. Bibcode:1981Sci...211..341L. doi:10.1126/science.211.4480.341. PMID 17748254.
- Tanner, N.M., "On Becoming Human", Cambridge University Press (Cambridge), (1981)
- Wescott, R.W. (1967). "Hominid Uprightness and Primate Display". American Anthropologist. 69: 738. doi:10.1525/aa.1967.69.6.02a00110.
- Wheeler, P. E. (1984) "The Evolution of Bipedality and Loss of Functional Body Hair in Hominoids." Journal of Human Evolution, 13, 91-98,
- Vrba, E. (1993). "The Pulse that Produced Us.". Natural History. 102 (5): 47–51.
External links
- The Origin of Bipedalism
- Human Timeline (Interactive) – Smithsonian, National Museum of Natural History (August 2016).