Chitosan
 | |
| Names | |
|---|---|
| Other names
Poliglusam; Deacetylchitin; Poly-(D)glucosamine; BC; Chitopearl; Chitopharm; Flonac; Kytex | |
| Identifiers | |
| 9012-76-4 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 64870 |
| ECHA InfoCard | 100.122.259 |
| |
| |
| Related compounds | |
| Related compounds |
D-glucosamine and N-acetylglucosamine (monomers) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
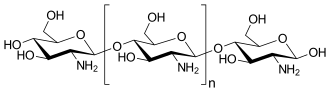
Chitosan /ˈkaɪtəsæn/ is a linear polysaccharide composed of randomly distributed β-(1→4)-linked D-glucosamine (deacetylated unit) and N-acetyl-D-glucosamine (acetylated unit). It is made by treating the chitin shells of shrimp and other crustaceans with an alkaline substance, like sodium hydroxide.
Chitosan has a number of commercial and possible biomedical uses. It can be used in agriculture as a seed treatment and biopesticide, helping plants to fight off fungal infections. In winemaking, it can be used as a fining agent, also helping to prevent spoilage. In industry, it can be used in a self-healing polyurethane paint coating. In medicine, it may be useful in bandages to reduce bleeding and as an antibacterial agent; it can also be used to help deliver drugs through the skin.
More controversially, chitosan has been asserted to have use in limiting fat absorption, which would make it useful for dieting, but there is evidence against this.
Other uses of chitosan that have been researched include use as a soluble dietary fiber.
Manufacture and properties
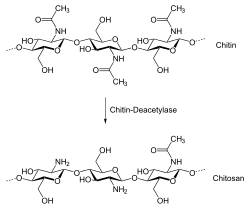

Chitosan is produced commercially by deacetylation of chitin, which is the structural element in the exoskeleton of crustaceans (such as crabs and shrimp) and cell walls of fungi. The degree of deacetylation (%DD) can be determined by NMR spectroscopy, and the %DD in commercial chitosans ranges from 60 to 100%. On average, the molecular weight of commercially produced chitosan is between 3800 and 20,000 Daltons. A common method for the synthesis of chitosan is the deacetylation of chitin using sodium hydroxide in excess as a reagent and water as a solvent. The reaction occurs in two stages under first-order kinetic control. Activation energy for the first step is higher than the second; its value is an estimated 48.76 kJ/mol at 25–120 °C.[2] This reaction pathway, when allowed to go to completion (complete deacetylation) yields up to 98% product.[3]
The amino group in chitosan has a pKa value of ~6.5, which leads to a protonation in acidic to neutral solution with a charge density dependent on pH and the %DA-value. This makes chitosan water-soluble and a bioadhesive which readily binds to negatively charged surfaces[4][5] such as mucosal membranes. Chitosan enhances the transport of polar drugs across epithelial surfaces, and is biocompatible and biodegradable. It is not approved by FDA for drug delivery though. Purified quantities of chitosans are available for biomedical applications.
Chitosan and its derivatives, such as trimethylchitosan (where the amino group has been trimethylated), have been used in nonviral gene delivery. Trimethylchitosan, or quaternised chitosan, has been shown to transfect breast cancer cells, with increased degree of trimethylation increasing the cytotoxicity; at approximately 50% trimethylation, the derivative is the most efficient at gene delivery. Oligomeric derivatives (3–6 kDa) are relatively nontoxic and have good gene delivery properties.[6]
Usage
Agricultural and horticultural use
The agricultural and horticultural uses for chitosan, primarily for plant defense and yield increase, are based on how this glucosamine polymer influences the biochemistry and molecular biology of the plant cell. The cellular targets are the plasma membrane and nuclear chromatin. Subsequent changes occur in cell membranes, chromatin, DNA, calcium, MAP Kinase, oxidative burst, reactive oxygen species, callose pathogenesis-related (PR) genes and phytoalexins.[7]
Natural biocontrol and elicitor
In agriculture, chitosan is typically used as a natural seed treatment and plant growth enhancer, and as an ecologically friendly biopesticide substance that boosts the innate ability of plants to defend themselves against fungal infections.[8] The natural biocontrol active ingredients, chitin/chitosan, are found in the shells of crustaceans, such as lobsters, crabs, and shrimp, and many other organisms, including insects and fungi. It is one of the most abundant biodegradable materials in the world.
Degraded molecules of chitin/chitosan exist in soil and water. Chitosan applications for plants and crops are regulated by the EPA, and the USDA National Organic Program regulates its use on organic certified farms and crops.[9] EPA-approved, biodegradable chitosan products are allowed for use outdoors and indoors on plants and crops grown commercially and by consumers.[10]
The natural biocontrol ability of chitosan should not be confused with the effects of fertilizers or pesticides upon plants or the environment. Chitosan active biopesticides represent a new tier of cost-effective biological control of crops for agriculture and horticulture.[11] The biocontrol mode of action of chitosan elicits natural innate defense responses within plant to resist insects, pathogens, and soil-borne diseases when applied to foliage or the soil.[12] Chitosan increases photosynthesis, promotes and enhances plant growth, stimulates nutrient uptake, increases germination and sprouting, and boosts plant vigor. When used as seed treatment or seed coating on cotton, corn, seed potatoes, soybeans, sugar beets, tomatoes, wheat and many other seeds, it elicits an innate immunity response in developing roots which destroys parasitic cyst nematodes without harming beneficial nematodes and organisms.[13][14]
Agricultural applications of chitosan can reduce environmental stress due to drought and soil deficiencies, strengthen seed vitality, improve stand quality, increase yields, and reduce fruit decay of vegetables, fruits and citrus crops .[15] Horticultural application of chitosan increases blooms and extends the life of cut flowers and Christmas trees. The US Forest Service has conducted research on chitosan to control pathogens in pine trees[16][17] and increase resin pitch outflow which resists pine beetle infestation.[18]

Chitosan has a rich history of being researched for applications in agriculture and horticulture dating back to the 1980s.[19] By 1989, chitosan salt solutions were applied to crops for improved freeze protection or to crop seed for seed priming.[20] Shortly thereafter, chitosan salt received the first ever biopesticide label from the EPA, then followed by other intellectual property applications.
Chitosan has been used to protect plants in space, as well, exemplified by NASA's experiment to protect adzuki beans grown aboard the space shuttle and Mir space station in 1997 (see photo left).[21] NASA results revealed chitosan induces increased growth (biomass) and pathogen resistance due to elevated levels of β-(1→3)-glucanase enzymes within plant cells. NASA confirmed chitosan elicits the same effect in plants on earth.[22]
Nontoxic, low molecular weight chitosan polymer solutions appear to be safe enough for broad-spectrum agricultural and horticultural uses.[23][24] In 2008, the EPA approved natural broad-spectrum elicitor status for an ultralow molecular active ingredient of 0.25% chitosan.[25]
A natural chitosan elicitor solution for agriculture and horticultural uses was granted an amended label for foliar and irrigation applications by the EPA in 2009.[15] Given its low potential for toxicity and abundance in the natural environment, chitosan does not harm people, pets, wildlife, or the environment when used according to label directions.[26][27][28] The US Forest Service tested chitosan as an ecofriendly biopesticide to prearm pine trees to defend themselves against mountain pine beetles.
Water filtration
Chitosan can also be used in water processing engineering as a part of a filtration process. Chitosan causes the fine sediment particles to bind together, and is subsequently removed with the sediment during sand filtration. It also removes phosphorus, heavy minerals, and oils from the water. Chitosan is an important additive in the filtration process. Sand filtration apparently can remove up to 50% of the turbidity alone, while the chitosan with sand filtration removes up to 99% turbidity.[29] Chitosan has been used to precipitate caseins from bovine milk and cheese making.[30][31]
Chitosan is also useful in other filtration situations, where one may need to remove suspended particles from a liquid. In combination with bentonite, gelatin, silica gel, isinglass, or other fining agents, it is used to clarify wine, mead, and beer. Added late in the brewing process, chitosan improves flocculation, and removes yeast cells, fruit particles, and other detritus that cause hazy wine. Chitosan combined with colloidal silica is becoming a popular fining agent for white wines, because chitosan does not require acidic tannins (found primarily in red wines) with which to flocculate.[32]
Bioprinting

Bioinspired materials, a manufacturing concept inspired by natural nacre, shrimp carapace or insect cuticles,[34][35][36] has led to development of bioprinting methods to manufacture large scale consumer objects using chitosan.[37][38] This method is based on replicating the molecular arrangement of chitosan from natural materials into fabrication methods, such as injection molding or mold casting.[39] Once discarded, chitosan-constructed objects are biodegradable and non-toxic.[40] The method is used to engineer and bioprint human organs or tissues.[41][42]
Pigmented chitosan objects can be recycled,[43] with the option of reintroducing or discarding the dye at each recycling step, enabling reuse of the polymer independently of colorants.[44][45] Unlike other plant-based bioplastics (e.g. cellulose, starch), the main natural sources of chitosan are from marine environments and do not compete for land or other human resources.[46][33]
The fabrication of large objects with chitosan applies concepts from Shrilk, a chitosan material that replicates insect cuticle and a fibroin protein from silk for uses in bioengineering.[47][48]
Winemaking and fungal source chitosan
Chitosan has a long history for use as a fining agent in winemaking.[49][50] Fungal source chitosan has shown an increase in settling activity, reduction of oxidized polyphenolics in juice and wine, chelation and removal of copper (post-racking) and control of the spoilage yeast Brettanomyces. These products and uses are approved for European use by the EU and OIV standards.[51]
Potential industrial uses
Scientists have developed a polyurethane coating that heals its own scratches when exposed to sunlight.[52][53] The self-healing coating uses chitosan incorporated into traditional polymer materials, such as those used in coatings on cars to protect paint. When a scratch damages the chemical structure, the chitosan responds to sunshine by forming chemical chains that bond with other materials in the substance, eventually smoothing the scratch. The process can take less than an hour.[54]
The polymer can only repair itself in the same spot once, and would not work after repeated scratches.[54] Whether this technology can be applied to industrial materials, however, depends on a number of factors, such as long-term persistence of the repair, stiffness and heat resistance of the coating.[55]
Biomedical uses
Chitosan's properties allow it to rapidly clot blood, and has recently gained approval in the United States[56] and Europe for use in bandages and other hemostatic agents. Chitosan hemostatic products have been shown in testing by the U.S. Marine Corps to quickly stop bleeding and to reduce blood loss, and result in 100% survival of otherwise lethal arterial wounds in swine.[57] Chitosan hemostatic products reduce blood loss in comparison to gauze dressings and increase patient survival.[58] Chitosan hemostatic products have been sold to the U.S. Army and are currently used by the UK military. Both the US and UK have already used the bandages on the battlefields of Iraq and Afghanistan.[59] Chitosan is hypoallergenic and has natural antibacterial properties, which further support its use in field bandages.[60] Chitosan's hemostatic properties also allow it to reduce pain by blocking nerve endings.[61]
Chitosan hemostatic agents are often chitosan salts made from mixing chitosan with an organic acid (such as succinic or lactic acid).[62] The hemostatic agent works by an interaction between the cell membrane of erythrocytes (negative charge) and the protonated chitosan (positive charge) leading to involvement of platelets and rapid thrombus formation.[63] The chitosan salts can be mixed with other materials to make them more absorbent (such as mixing with alginate),[64] or to vary the rate of solubility and bioabsorbability of the chitosan salt.[62] The chitosan salts are biocompatible and biodegradable making them useful as absorbable haemostats. The protonated chitosan is broken down by lysozyme in the body to glucosamine [63] and the conjugate base of the acid (such as lactate or succinate) are substances naturally found in the body. The chitosan salt may be placed on an absorbable backing.[65] The absorbable backing may be synthetic (for instance made from existing absorbable suture materials e.g. Tephaflex polymer) or natural (e.g. cellulose or gelled/solidified honey). In addition to salts, hydrogel-based chitosan bandages have been developed to treat burn wounds. Burns are similar to other wounds, but are problematic because they are associated with membrane destabilization, energy depletion, and hypoxia, all of which can cause severe tissue necrosis if not treated properly or quickly enough. Chitosan-gelation bandages using nanofibrin have been shown to be more durable than ointments, while still allowing gas exchange at the cell surface.[66]
Chitosan's properties also allow it to be used in transdermal drug delivery; it is mucoadhesive in nature, reactive (so it can be produced in many different forms), and most importantly, has a positive charge under acidic conditions. This positive charge comes from protonation of its free amino groups. Lack of a positive charge means chitosan is insoluble in neutral and basic environments. However, in acidic environments, protonation of the amino groups leads to an increase in solubility. The implications of this are very important to biomedical applications. This molecule will maintain its structure in a neutral environment, but will solubilize and degrade in an acidic environment. This means chitosan can be used to transport a drug to an acidic environment, where the chitosan packaging will then degrade, releasing the drug to the desired environment.[67] One example of this drug delivery has been the transport of insulin.[68]
Chitosan can also be combined with other materials. For example, a composite with hydroxyapatite was effective as a temporary post-operation bone filler, which was gradually biodegraded and replaced by native bone tissue.[69]
Research
Weight loss
Supposed to limit fat absorption in the body, chitosan may be sold in tablet form as a "fat binder". A Cochrane meta-analysis[70] that evaluated clinical trials performed with dietary chitosan over a minimum of four weeks, found that body weight, blood pressure and cholesterol-related parameters changed only in some low-quality trials, indicating a minor effect on body weight. Other higher quality trials indicated no significant effect of chitosan and no clinical justification for advising overweight patients to take chitosan supplements.[70]
In an experimental model of the digestive tract, chitosan was shown to interact with oil, which inhibited duodenal absorption and enhanced lipid excretion.[71] However, the mechanism of interaction between chitosan and fat remained poorly understood and clinically unproven.[72] In mice, dietary ingestion of chitosan did not depress iron, zinc or copper levels.[73]
The U.S. Food and Drug Administration (FDA) issued warning letters to supplement retailers who made inappropriate claims about the supposed health benefits of using chitosan.[74]
Chitosan is under research for several potential dietary or clinical applications:[75]
- As a soluble dietary fiber, it may increase gastrointestinal lumen viscosity and retard emptying of the stomach, creating a sense of satiety.[76][77]
- It alters bile acid composition, increasing the excretion of sterols and reducing the digestibility of ileal fats.[78][79][80] It is unclear how chitosan does this, but the currently favored hypotheses involve the increase of intestinal viscosity or bile acid-binding capacity.[81]
- Chitosan is relatively insoluble in water, making it a viscous dietary fiber possibly inhibiting absorption of dietary lipids.[81]
Food preservation
In 2016 researchers announced a chitosan-based plastic wrap that doubles the shelf life of some foods. The plastic also included grapefruit seed extract, which has antibacterial and antifungal properties, and is an antioxidant, antiseptic and anti-viral. The film blocked the transmission of ultraviolet light — slowing oxidation and photochemical deterioration. The plastic can use raw ingredients that would otherwise be discarded, and biodegrades once discarded.[82]
References
- ↑ Shahidi, Fereidoon; Synowiecki, Jozef (1991). "Isolation and characterization of nutrients and value-added products from snow crab (Chionoecetes opilio) and shrimp (Pandalus borealis) processing discards". Journal of Agricultural and Food Chemistry. 39 (8): 1527–32. doi:10.1021/jf00008a032.
- ↑ Ahlafi, Hammou; et al. (2013). "Kinetics of N-Deacetylation of Chitin Extracted from Shrimp Shells Collected from Coastal Area of Morocco" (PDF). medjchem.com. Mediterranean Journal of Chemistry. Retrieved 19 October 2015.
- ↑ Peniston, Quintin P. et al. (25 March 1980) "Process for the manufacture of chitosan" U.S. Patent 4,195,175
- ↑ Dong Woog Lee; et al. (2013). "Strong adhesion and cohesion of chitosan in aqueous solutions". Langmuir. 29 (46): 14222–14229. doi:10.1021/la403124u.
- ↑ Chanoong Lim; Dong Woog Lee; et al. (2015). "Contact time- and pH-dependent adhesion and cohesion of low molecular weight chitosan coated surfaces". Carbohydrate Polymers. 117 (6): 887–894. doi:10.1016/j.carbpol.2014.10.033.
- ↑ Kean, Thomas; Roth, Susanne; Thanou, Maya (2005). "Trimethylated chitosans as non-viral gene delivery vectors: Cytotoxicity and transfection efficiency". Journal of Controlled Release. 103 (3): 643–53. doi:10.1016/j.jconrel.2005.01.001. PMID 15820411.
- ↑ Hadwiger, Lee A. (2013). "Multiple effects of chitosan on plant systems: Solid science or hype". Plant Science. 208: 42–9. doi:10.1016/j.plantsci.2013.03.007. PMID 23683928.
- ↑ Linden, James C.; Stoner, Richard J.; Knutson, Kenneth W.; Gardner-Hughes, Cecilie A. (2000). "Organic disease control elicitors". Agro Food Industry Hi-Tech. 11 (5): 32–4.
- ↑ "USDA NOP and EPA Rule on Chitosan, Federal Register/Vol. 72, No. 236/Monday, December 10, 2007/Rules and Regulation" (PDF). Archived from the original (PDF) on 11 December 2008.
- ↑ "Chitin and Chitosan Final Registration Review Decision, Document ID: EPA-HQ-OPP-2007-0566-0019". Regulations.gov. 11 December 2008. pp. 10–15.
- ↑ Goosen, Mattheus F. A. (1 June 1996). Applications of Chitan and Chitosan. CRC Press. pp. 132–9. ISBN 978-1-56676-449-0.
- ↑ Linden, J.C.; Stoner, R.J. (2005). "Proprietary Elicitor Affects Seed Germination and Delays Fruit Senescence" (PDF). Journal of Food, Agriculture & Environment.
- ↑ "Smiley R., Cook R.J., Pauliz T., Seed Treatment for Sample Cereal Grains Oregon State University, 2002, EM 8797" (PDF). Archived from the original (PDF) on 5 September 2006.
- ↑ "Stoner R., Linden J., Micronutrient elicitor for treating nematodes in field crops, 2006, Patent Pending, Pub. no.: US 2008/0072494 A1".
- 1 2 Linden, J. C.; Stoner, R. J. (2007). "Pre-harvest application of proprietary elicitor delays fruit senescence". Advances in Plant Ethylene Research. pp. 301–2. doi:10.1007/978-1-4020-6014-4_65. ISBN 978-1-4020-6013-7.
- ↑ Mason, Mary E.; Davis, John M. (1997). "Defense Response in Slash Pine: Chitosan Treatment Alters the Abundance of Specific mRNAs". Molecular Plant-Microbe Interactions. 10 (1): 135–7. doi:10.1094/MPMI.1997.10.1.135. PMID 9002276.
- ↑ Klepzig, Kier D.; Walkinshaw, Charles H. (2003). "Cellular response of loblolly pine to wound inoculation with bark beetle-associated fungi and chitosan".
- ↑ O'Toole, Erin (10 September 2009). "Solution for Pine Bark Beetles May Help Front Range Trees". NPR Morning Edition – KUNC 91.5 FM Greeley, CO.
- ↑ Croteau, R.; Gurkewitz, S.; Johnson, M. A.; Fisk, H. J. (1987). "Biochemistry of Oleoresinosis : Monoterpene and Diterpene Biosynthesis in Lodgepole Pine Saplings Infected with Ceratocystis clavigera or Treated with Carbohydrate Elicitors". Plant Physiology. 85 (4): 1123–8. doi:10.1104/pp.85.4.1123. PMC 1054405
 . PMID 16665815.
. PMID 16665815. - ↑ "Treatment of Plants with Chitosan Salts, 1989, Patent WO/1989/007395".
- ↑ Stoner, R. (2006). "Progressive Plant Growing Has Business Blooming, Environmental and Agricultural Resources". NASA. pp. 68–71..
- ↑ Linden, James C.; Stoner, Richard J. (21 October 2008). "YEA! Elicitor Response Comparison to Chitin / Chitosan in Mung Bean and Adzuki Bean Germination Experiments" (PDF).
- ↑ "BIOPOLYMERS Making Materials Nature's Way".
- ↑ "SeedQuest Press Release: AgriHouse Acquires DCV Chitosan IP and Patents".
- ↑ "Chitin/Chitosan, Farnesol/Nerolidol and Nosema locustae Final Registration Review Decision". Federal Register Notice of Availability. EPA. 73 (248). 24 December 2008.
- ↑ "Chitosan Exemption from the Requirement of a Tolerance". US Environmental Protection Agency.
- ↑ "Control Strategies to reduce postharvest decay of fresh fruits and vegetables". USDA.gov.
- ↑ "Chitosan; Poly-D-glucosamine (128930) Fact Sheet". US Environmental Protection Agency. 2 May 2006. Retrieved 10 July 2006.
- ↑ Alan Woodmansey (Highway Engineer) (19 March 2002). "Chitosan Treatment of Sediment Laden Water – Washington State I-90 Issaquah Project". Federal Highway Administration. U.S. Department of Transportation. Retrieved 10 July 2006.
- ↑ Ausar, Salvador F; Passalacqua, Nancy; Castagna, Leonardo F; Bianco, Ismael D; Beltramo, Dante M (2002). "Growth of milk fermentative bacteria in the presence of chitosan for potential use in cheese making". International Dairy Journal. 12 (11): 899–906. doi:10.1016/S0958-6946(02)00114-0.
- ↑ Ausar SF1, Bianco ID, Badini RG, Castagna LF, Modesti NM, Landa CA, Beltramo DM (2001). "Characterization of casein micelle precipitation by chitosans". J Dairy Sci. 84 (2): 361–9. doi:10.3168/jds.S0022-0302(01)74485-2. PMID 11233020.
- ↑ Rayner, Terry. "Fining and Clarifying Agents". Archived from the original on 16 June 2006. Retrieved 18 July 2006.
- 1 2 Fernandez J, Ingber D (February 2014). "Manufacturing of large-scale functional objects using biodegradable chitosan bioplastic". Macromolecular Materials and Engineering. doi:10.1002/mame.201300426.
- ↑ Tampieri, A; Celotti, G; Landi, E; Sandri, M; Roveri, N; Falini, G (2003). "Biologically inspired synthesis of bone-like composite: Self-assembled collagen fibers/hydroxyapatite nanocrystals". Journal of Biomedical Materials Research. 67 (2): 618–25. doi:10.1002/jbm.a.10039. PMID 14566805.
- ↑ Tampieri, A; Celotti, G; Landi, E (2005). "From biomimetic apatites to biologically inspired composites". Analytical and Bioanalytical Chemistry. 381 (3): 568–76. doi:10.1007/s00216-004-2943-0. PMID 15696277.
- ↑ Cheng, Q; Jiang, L; Tang, Z (2014). "Bioinspired layered materials with superior mechanical performance". Accounts of Chemical Research. 47 (4): 1256–66. doi:10.1021/ar400279t. PMID 24635413.
- ↑ Tajik, H; Moradi, M; Rohani, S. M.; Erfani, A. M.; Jalali, F. S. (2008). "Preparation of chitosan from brine shrimp (Artemia urmiana) cyst shells and effects of different chemical processing sequences on the physicochemical and functional properties of the product". Molecules (Basel, Switzerland). 13 (6): 1263–74. PMID 18596653.
- ↑ Fernandez, J. G.; Ingber, D. E. (2012). "Unexpected strength and toughness in chitosan-fibroin laminates inspired by insect cuticle". Advanced Materials. 24 (4): 480–4. doi:10.1002/adma.201104051. PMID 22162193.
- ↑ Wyss Institute Communications (May 2014). "Promising solution to plastic pollution". Harvard Gazette, Harvard University, Boston, MA. Retrieved 23 May 2014.
- ↑ Shukla, S. K.; Mishra, A. K.; Arotiba, O. A.; Mamba, B. B. (2013). "Chitosan-based nanomaterials: A state-of-the-art review". International Journal of Biological Macromolecules. 59: 46–58. doi:10.1016/j.ijbiomac.2013.04.043. PMID 23608103.
- ↑ Lee, J. Y.; Choi, B.; Wu, B.; Lee, M. (2013). "Customized biomimetic scaffolds created by indirect three-dimensional printing for tissue engineering". Biofabrication. 5 (4): 045003. doi:10.1088/1758-5082/5/4/045003. PMID 24060622.
- ↑ Selko A (6 March 2014). "Manufacturing 3-D objects just got easier with new bioplastic". Industry Week. Retrieved 24 May 2014.
- ↑ Fernandez, J. G.; Ingber, D. E. (2014). "Manufacturing of Large-Scale Functional Objects Using Biodegradable Chitosan Bioplastic". Macromolecular Materials and Engineering: n/a. doi:10.1002/mame.201300426.
- ↑ "Manufacturing a solution to planet-clogging plastics". Hansjorg Wyss Institute for Biologically Inspired Engineering, Harvard University. 3 March 2014. Retrieved 5 June 2014.
- ↑ Zhao, Y; Xie, Z; Gu, H; Zhu, C; Gu, Z (2012). "Bio-inspired variable structural color materials". Chemical Society Reviews. 41 (8): 3297–317. doi:10.1039/c2cs15267c. PMID 22302077.
- ↑ "Chitosan bioplastic". Wyss Institute Communications, Hansjorg Wyss Institute for Biologically Inspired Engineering, Harvard University. 2014. Retrieved 24 May 2014.
- ↑ "Harvard researchers develop bioplastic made from shrimp shells". Fox News.
- ↑ Sample I (15 April 2014). "Five wonder materials that could change the world". The Guardian. Retrieved 24 May 2014.
- ↑ Chorniak J (October 2007). "A clearer understanding of fining agents". Wine Maker Magazine. Retrieved 24 May 2014.
- ↑ Quintela, S; Villarán, M. C.; López De Armentia, I; Elejalde, E (2012). "Ochratoxin a removal from red wine by several oenological fining agents: Bentonite, egg albumin, allergen-free adsorbents, chitin and chitosan". Food Additives & Contaminants: Part A. 29 (7): 1168–74. doi:10.1080/19440049.2012.682166. PMID 22545592.
- ↑ Escudero-Abarca, Blanca I.; Escudero-Abarca, M. Guadalupe; Aguilar-Uscanga, Patricia M.; Hayward-Jones, Patricia; Mendoza, Mario; Ramírez, Leticia (2004). "Selective antimicrobial action of chitosan against spoilage yeasts in mixed culture fermentations". Journal of Industrial Microbiology and Biotechnology. 31 (1): 16–22. doi:10.1007/s10295-004-0112-2. PMID 14747932.
- ↑ Steenhuysen J (13 March 2009). "Coating makes scratches on cars disappear". Reuters. Retrieved 24 May 2014.
- ↑ Burkhard, J. A.; Wuitschik, G; Rogers-Evans, M; Müller, K; Carreira, E. M. (2010). "Oxetanes as versatile elements in drug discovery and synthesis". Angewandte Chemie International Edition. 49 (48): 9052–67. doi:10.1002/anie.200907155. PMID 21031377.
- 1 2 Ghosh, B; Urban, MW (2009). "Self-Repairing Oxetane-Substituted Chitosan Polyurethane Networks". Science. 323 (5920): 1458–60. doi:10.1126/science.1167391. PMID 19286550.
- ↑ Kleiner K (12 March 2009). "Crab chemical could give cars a self-healing 'shell'". New Scientist-Tech. Retrieved 24 May 2014.
- ↑ Zhang, Yin-Juan; Gao, Bo; Liu, Xi-Wen (2015). "Topical and effective hemostatic medicines in the battlefield". Int J Clin Exp Med. 8 (1): 10–19. PMC 4358424
 . PMID 25784969.
. PMID 25784969. - ↑ Brown, Mark A.; Daya, Mohamud R.; Worley, Joseph A. (2009). "Experience with Chitosan Dressings in a Civilian EMS System". The Journal of Emergency Medicine. 37 (1): 1–7. doi:10.1016/j.jemermed.2007.05.043. PMID 18024069.
- ↑ Pusateri, Anthony E.; McCarthy, Simon J.; Gregory, Kenton W.; Harris, Richard A.; Cardenas, Luis; McManus, Albert T.; Goodwin, Cleon W. (2003). "Effect of a Chitosan-Based Hemostatic Dressing on Blood Loss and Survival in a Model of Severe Venous Hemorrhage and Hepatic Injury in Swine". The Journal of Trauma: Injury, Infection, and Critical Care. 54 (1): 177–82. doi:10.1097/00005373-200301000-00023. PMID 12544915.
- ↑ Kheirabadi BS; et al. (18 August 2004). "Development of Hemostatic Dressings for Use in Military Operations" (PDF). Symposium on Combat Casualty Care in Ground Based Tactical Situations: Trauma Technology and Emergency Medical Procedures, St. Petersburg Beach, US. Retrieved 5 June 2014.
- ↑ Kevin McCue (3 March 2003). "New Bandage Uses Biopolymer". Chemistry.org. American Chemical Society. Archived from the original on 28 November 2005. Retrieved 10 July 2006.
- ↑ Sudheesh Kumar, P.T.; et al. "Flexible, micro-porous chitosan–gelatin hydrogel/ nanofibrin composite bandages for treating burn wounds". Royal Society of Chemistry. RSC Adv.,2014,4,65081. Retrieved 19 October 2015.
- 1 2 US patent 8106030, Hardy, Craig; Johnson, Lee and Luksch, Paul, "Hemostatic Material", issued 2012-01-31
- 1 2 Baldrick, Paul (2010). "The safety of chitosan as a pharmaceutical excipient". Regulatory Toxicology and Pharmacology. 56 (3): 290–9. doi:10.1016/j.yrtph.2009.09.015. PMID 19788905.
- ↑ US patent 5836970, Pandit, Abhay S., "Hemostatic Wound Dressing", issued 1998-11-17
- ↑ US application 2011052665, Hardy, Craig; Darby, Andrew and Eason, Guy, "Hemostatic Material"
- ↑ Sudeesh Kumar, P.T.; et al. "Flexible, micro-porous chitosan–gelatin hydrogel/ nanofibrin composite bandages for treating burn wounds". Royal Society of Chemistry. RSC Adv.,2014,4,65081. Retrieved 19 October 2015.
- ↑ Sadigh-Eteghad, Saeed; Talebi, Mahnaz; Farhoudi, Mehdi; Mahmoudi, Javad; Reyhani, Bahram (2013). "Effects of Levodopa loaded chitosan nanoparticles on cell viability and caspase-3 expression in PC12 neural like cells". Neurosciences (Riyadh). 18 (3): 281–283. PMID 23887222.
- ↑ Agnihotri, Sunil A.; Mallikarjuna, Nadagouda N.; Aminabhavi, Tejraj M. (2004). "Recent advances on chitosan-based micro- and nanoparticles in drug delivery". Journal of Controlled Release. 100 (1): 5–28. doi:10.1016/j.jconrel.2004.08.010. PMID 15491807.
- ↑ Danilchenko, S.N.; Kalinkevich, O.V.; Pogorelov, M.V. (2009). "Chitosan–hydroxyapatite composite biomaterials made by a one step co-precipitation method: preparation, characterization and in vivo tests". Journal of Biological Physics and Chemistry. 9 (3): 119–126. doi:10.4024/22DA09A.jbpc.09.03.
- 1 2 Jull, Andrew B; Ni Mhurchu, Cliona; Bennett, Derrick A; Dunshea-Mooij, Christel AE; Rodgers, Anthony (2008). Jull, Andrew B, ed. "Chitosan for overweight or obesity". Cochrane Database of Systematic Reviews (3): CD003892. doi:10.1002/14651858.CD003892.pub3. PMID 18646097.
- ↑ Rodríguez, María Susana; Albertengo, Liliana Elena (2005). "Interaction between Chitosan and Oil under Stomach and Duodenal Digestive Chemical Conditions". Bioscience, Biotechnology, and Biochemistry. 69 (11): 2057–62. doi:10.1271/bbb.69.2057. PMID 16306685.
- ↑ Gades, Matthew D.; Stern, Judith S. (2003). "Chitosan Supplementation and Fecal Fat Excretion in Men". Obesity. 11 (5): 683–8. doi:10.1038/oby.2003.97. PMID 12740459.
- ↑ Zeng, Lintao; Qin, Caiqin; He, Guanghui; Wang, Wei; Li, Wei; Xu, Dongsheng (2008). "Effect of dietary chitosans on trace iron, copper and zinc in mice". Carbohydrate Polymers. 74 (2): 279–82. doi:10.1016/j.carbpol.2008.02.022.
- ↑ "Warning Letter for Chitosan Weight Loss Products". FDA.gov.
- ↑ Knorr, D. (January 1991). "Recovery and utilization of chitin and chitosan in food processing waste management". Food Technology. 45 (1): 114–22.
- ↑ Yao, H. T.; Chiang, M. T. (2006). "Chitosan shifts the fermentation site toward the distal colon and increases the fecal short-chain fatty acids concentrations in rats". International Journal for Vitamin and Nutrition Research. 76 (2): 57–64. doi:10.1024/0300-9831.76.2.57. PMID 16941416.
- ↑ Van Bennekum, A. M.; Nguyen, D. V.; Schulthess, G; Hauser, H; Phillips, M. C. (2005). "Mechanisms of cholesterol-lowering effects of dietary insoluble fibres: Relationships with intestinal and hepatic cholesterol parameters". The British journal of nutrition. 94 (3): 331–7. doi:10.1079/bjn20051498. PMID 16176602.
- ↑ Fukada, Yasuhiko; Kimura, Koji; Ayaki, Yoshikazu (1991). "Effect of chitosan feeding on intestinal bile acid metabolism in rats". Lipids. 26 (5): 395–9. doi:10.1007/BF02537206. PMID 1895888.
- ↑ Maezaki, Yuji; Tsuji, Keisuke; Nakagawa, Yasue; Kawai, Yoshiyuki; Akimoto, Makoto; Tsugita, Takashi; Takekawa, Wataru; Terada, Atsushi; Hara, Hiroyoshi; Mitsuoka, Tomotari (1993). "Hypocholesterolemic Effect of Chitosan in Adult Males". Bioscience, Biotechnology, and Biochemistry. 57 (9): 1439–44. doi:10.1271/bbb.57.1439.
- ↑ Razdan, A.; Pettersson, D. (2007). "Effect of chitin and chitosan on nutrient digestibility and plasma lipid concentrations in broiler chickens". British Journal of Nutrition. 72 (2): 277–88. doi:10.1079/BJN19940029. PMID 7947645.
- 1 2 Furda, Ivan (1990). "Interaction of Dietary Fiber with Lipids — Mechanistic Theories and their Limitations". New Developments in Dietary Fiber. Advances in Experimental Medicine and Biology. 270. pp. 67–82. doi:10.1007/978-1-4684-5784-1_7. ISBN 978-1-4684-5786-5. PMID 1964019.
- ↑ Coxworth, Ben (23 February 2016). "Shrimp-shell film doubles shelf life of foods". www.gizmag.com. Retrieved 25 February 2016.
External links
- International research project Nano3Bio, focused on tailor-made biotechnological production of chitosans (funded by the European Union)