Amylose
 | |
| Identifiers | |
|---|---|
| 9005-82-7 | |
| ChEBI | CHEBI:28102 |
| ECHA InfoCard | 100.029.702 |
| UNII | 7TDQ74Y18L |
| Properties | |
| variable | |
| Molar mass | variable |
| Appearance | white powder |
| Hazards | |
| NFPA 704 | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
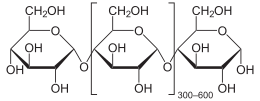
Amylose is a helical polymer made of α-D-glucose units, bound to each other through α(1→4) glycosidic bonds.
This polysaccharide is one of the two components of starch, making up approximately 20-30% of the structure. The other component is amylopectin, which makes up 70–80% of the structure.[1]
Because of its tightly packed structure, amylose is more resistant to digestion than other starch molecules and is therefore an important form of resistant starch, which has been found to be an effective prebiotic.[2]
Structure
Amylose is made up of α(1→4) bound glucose molecules. The carbon atoms on glucose are numbered, starting at the aldehyde (C=O) carbon, so, in amylose, the 1-carbon on one glucose molecule is linked to the 4-carbon on the next glucose molecule (α(1→4) bonds).[3] The structural formula of amylose is pictured at right. The number of repeated glucose subunits (n) is usually in the range of 300 to 3000, but can be many thousands.
There are three main forms of amylose chains can take. It can exist in a disordered amorphous conformation or two different helical forms. It can bind with itself in a double helix (A or B form), or it can bind with another hydrophobic guest molecule such as iodine, a fatty acid, or an aromatic compound. This is known as the V form and is how amylopectin binds to amylose to form starch. Within this group, there are many different variations. Each is notated with V and then a subscript indicating the number of glucose units per turn. The most common is the V6 form, which has six glucose units a turn. V8 and possibly V7 forms exist as well. These provide an even larger space for the guest molecule to bind.[4]
This linear structure can have some rotation around the phi and psi angles, but for the most part bound glucose ring oxygens lie on one side of the structure. The α(1→4) structure promotes the formation of a helix structure, making it possible for hydrogen bonds to form between the oxygen atoms bound at the 2-carbon of one glucose molecule and the 3-carbon of the next glucose molecule.[5]
Physical properties
Because the long linear chains of amylose more readily crystallize than amylopectin (which has short, highly branched chains), high-amylose starch is more resistant to digestion.[6] Unlike amylopectin, amylose is not soluble in cold water.[7] It also reduces the crystallinity of amylopectin and how easily water can infiltrate the starch.[5] The higher the amylose content, the less expansion potential and the lower the gel strength for the same starch concentration. This can be countered partially by increasing the granule size.[8]
Fiber X-ray diffraction analysis coupled with computer-based structure refinement has found A-, B-, and C- polymorphs of amylose. Each form corresponds to either the A-, the B-, or the C- starch forms. A- and B- structures have different helical crystal structures and water contents, whereas the C- structure is a mixture of A- and B- unit cells, resulting in an intermediate packing density between the two forms.[9]
Function
Amylose is important in plant energy storage. It is less readily digested than amylopectin; however, because it is more linear than amylopectin, it takes up less space. As a result, it is the preferred starch for storage in plants. It makes up about 30% of the stored starch in plants, though the specific percentage varies by species. The digestive enzyme α-amylase is responsible for the breakdown of the starch molecule into maltotriose and maltose, which can be used as sources of energy.
Amylose is also an important thickener, water binder, emulsion stabilizer, and gelling agent in both industrial and food-based contexts. Loose helical amylose chains have a hydrophobic interior that can bind to hydrophobic molecules such as lipids and aromatic compounds. The one problem with this is that, when it crystallizes or associates, it can lose some stability, often releasing water in the process (syneresis). When amylose concentration is increased, gel stickiness decreases but gel firmness increases. When other things including amylopectin bind to amylose, the viscosity can be affected, but incorporating κ-carrageenan, alginate, xanthan gum, or low-molecular-weight sugars can reduce the loss in stability. The ability to bind water can add substance to food, possibly serving as a fat replacement.[10] For example, amylose is responsible for causing white sauce to thicken, but, upon cooling, some separation between the solid and the water will occur.
In a laboratory setting, it can act as a marker. Iodine molecules fit neatly inside the helical structure of amylose, binding with the starch polymer that absorbs certain known wavelengths of light. Hence, a common test is the iodine test for starch. Mix starch with a small amount of yellow iodine solution. In the presence of amylose, a blue-black color will be observed. The intensity of the color can be tested with a colorimeter, using a red filter to discern the concentration of starch present in the solution. It is also possible to use starch as an indicator in titrations involving iodine reduction.[11] It is also used in amylose magnetic beads and resin to separate maltose-binding protein[12]
Recent studies
High-amylose varieties of rice, the less sticky long-grain rice, have a much lower glycemic load, which could be beneficial for diabetics.
Researchers have identified the Granule Bound Starch Synthase (GBSS) as the enzyme that specifically elongates amylose during starch biosynthesis in plants.[13] The waxy locus in maize encodes for the GBSS protein.[13] Mutants lacking the GBSS protein produce starch containing only amylopectin, such as in waxy corn. In Arabidopsis leaves, another gene, encoding the Protein Targeting to STarch (PTST) protein, is required in addition to GBSS for amylose synthesis. Mutants lacking either protein produce starch without amylose.[14] GM potato cultivar Amflora by BASF Plant Science was developed to not produce amylose.
See also
- Amflora, genetically modified low amylose potato (high in amylopectin)
- Amylomaize, high amylose maize starch
- Russet Burbank potato, high amylose potato cultivar
Notes
- ↑ http://www.gmo-compass.org/eng/glossary/
- ↑ "Archived copy". Archived from the original on 2010-09-24. Retrieved 2010-07-02.
- ↑ Nelson, David , and Michael M. Cox. Principles of Biochemistry. 5th ed. New York: W. H. Freeman and Company , 2008.
- ↑ Cohen, R.; Orlova, Y.; Kovalev, M.; Ungar, Y.; Shimoni, E. (2008). "Structural and Functional Properties of Amylose Complexes with Genistein". Journal of Agricultural and Food Chemistry. 56 (11): 4212–4218. doi:10.1021/jf800255c. PMID 18489110.
- 1 2 "Archived copy". Archived from the original on 2012-01-14. Retrieved 2010-05-25.
- ↑ Birt DF, Boylston T, Hendrich S, Jane JL, Hollis J, Li L, McClelland J, Moore S, Phillips GJ, Rowling M, Schalinske K, Scott MP, Whitley EM (2013). "Resistant starch: promise for improving human health". Advances in Nutrition. 4 (6): 587–601. doi:10.3945/an.113.004325. PMC 3823506
 . PMID 24228189.
. PMID 24228189. - ↑ http://www.eric.ed.gov/ERICWebPortal/custom/portlets/recordDetails/detailmini.jsp?_nfpb=true&_&ERICExtSearch_SearchValue_0=EJ128481&ERICExtSearch_SearchType_0=no&accno=EJ128481
- ↑ (a) J-Y. Li and A-I. Yeh, Relationships between thermal, rheological characteristics and swelling power for various starches, J. Food Engineering 50 (2001) 141-148. (b) N. Singh, J. Singh, L. Kaur, N. Singh Sodhi and B. Singh Gill, Morphological, thermal and rheological properties of starches from different botanical sources, Food Chem. 81 (2003) 219-231.
- ↑ Sarko, A., and H.-C. H. Wu. 1978. The Crystal Structures of A-, B- and C-Polymorphs of Amylose and Starch. Starch 30: 73-78.
- ↑ H.-J. Chung, Q. Liu, Impact of molecular structure of amylopectin and amylose on amylose chain association during cooling, Carbohydr. Polymers 77 (2009) 807-815
- ↑ "Archived copy". Archived from the original on 2011-09-27. Retrieved 2010-05-25.
- ↑ "Archived copy". Archived from the original on 2010-01-08. Retrieved 2010-05-25.
- 1 2 http://www.annualreviews.org/doi/abs/10.1146/annurev.food.102308.124214
- ↑ http://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.1002080
References
- Zhong, F., Yokoyama, W.H., Wang, Q., Shoemaker, C.F. 2006. Rice Starch, Amylopectin, and Amylose: Molecular Weight and Solubility in Dimethyl Sulfoxide-Based Solvents. Journal of Agricultural and Food Chemistry. 10.1021:A-G.
External links
| Wikimedia Commons has media related to Amylose. |
