Cicutoxin
 | |
 | |
| Names | |
|---|---|
| IUPAC name
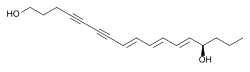
(8E,10E,12E,14R)-heptadeca-8,10,12-triene-4,6-diyne-1,14-diol | |
| Other names
Cicutoxin | |
| Identifiers | |
| 505-75-9 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL140367 |
| ChemSpider | 23215724 |
| |
| |
| Properties | |
| C17H22O2 | |
| Molar mass | 258.36 g·mol−1 |
| Density | 1.025 g/mL |
| Melting point | 54 °C (129 °F; 327 K) (prisms from ether & petroleum ether); 67 °C (crystals from ether & petroleum ether) |
| Boiling point | Decomposes above 35 °C (95 °F; 308 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Cicutoxin is a poisonous polyyne and alcohol found in various plants, such as the highly toxic water hemlock (Cicuta species). It is a natural product structurally related to the oenanthotoxin of hemlock water dropwort.
It causes death by disruption of the central nervous system. It is a potent, noncompetitive gamma-aminobutyric acid (GABA) receptor antagonist. In humans, cicutoxin rapidly produces symptoms of nausea, emesis and abdominal pain, typically within 60 minutes of ingestion. This can lead to tremors, seizures, and death.[1] LD50(mouse; i.p.) ~9 mg/kg[2]
History
The toxic effect of the plants that contains cicutoxin have been known for centuries.[3] As early as 1911, 27 cases of cicutoxin poisoning were known, 21 of the cases resulted in death. In some of these cases the plant was used for murder or execution.[4] 78 cases were reported in 1962, 33 of them resulted in death.[5] These days there are still cases of cicutoxin poisoning:[6]
-A child used the stem of a plant as a toy whistle and died of cicutoxin poisoning
-A 14-year-old boy died 20 hours after consuming a ‘wild carrot’
-In 1992, two brothers were foraging for wild ginseng, one of them ate three bites of a ‘ginseng’-root and the other one ingested one bite. The first brother died three hours after ingesting the root. His brother only suffered seizures and delirium, but had full recovery
-A family of five used a cicuta plant to relieve themselves of pruritus. The cicutoxin caused toxic symptoms and two of the children died.
All plants from the genus Cicuta contain cicutoxin, these plants are found in swampy, wet habitats in North America and parts of Europe. The Cicuta plants are often mistaken for edible roots such as parsnip, wild carrot or wild ginseng. All parts of the cicuta plants are poisonous, though the root is the most toxic part of the plant. 17 boys ingested parts of the plant, only those who consumed the root were experiencing seizures, the others who consumed the leaves and flowers were only unwell. The toxicity of the plants depends on various factors, such as seasonal variation, temperature, geographical location and soil conditions. Especially in the early spring the roots are the most toxic part of the plant. The roots contain their toxicity even after drying.[4] Cicutoxin was first isolated by C.A. Jacobsen in 1915. In 1953, the structure of the toxin was determined. The first synthesis of cicutoxin was reported in 1955, the researchers had a yield of only 4 percent. Given that they had no access to modern coupling reactions, this was a great accomplishment. The absolute configuration of cicutoxin was identified in 1999, they also discovered that the plants contain multiple congeners of cicutoxin.[7]
Structure and reactivity
The molecular formula for cicutoxin is C17H22O2. It is an aliphatic, highly unsaturated alcohol with two triple bonds, three double bonds and two hydroxyl groups. The natural form that occurs in plants is (R)-(−)-cicutoxin, but when cicutoxin is synthesized it is racemic mixture. There are also some closely related compounds that have almost the same structure cicutoxin such as Virol A and Virol C. When cicutoxin is isolated it is a yellowish oil. In the plant the compound is stable but when exposed to air, light or heat it becomes brown and cicutoxin is broken down.[8] Because cicutoxin breaks down so rapidly, research on the compound isn’t easy. Cicutoxin has a long carbon structure and few hydrophilic substituents which gives it hydrophobic characteristics. Hydrophobic and/or small molecules can be absorbed through the skin and for cicutoxin it has been proven to be absorbed through the skin of frogs.[9] There is also the example of the family that used a cicuta plant to relieve themselves of pruritus (also mentioned in the history) that shows cicutoxin can be absorbed through the skin.
Synthesis
The first article about the synthesis of cicutoxin was published in 1955. The racemic cicutoxin that was synthesized, turned out to be about twice as active as the natural material. In 2009 the synthesis of the natural polyenyne (R)-(–)-cicutoxin was described[7] (R)-(–)-cicutoxin can be synthesized in four linear steps, with the three key fragments: (R)-(–)-1-hexyn-3-ol (8), 1,4-diiodo-1,3-butadiene (9), and THP-protected 4,6-heptadiyn-1-ol (6). (R)-(–)-1-hexyn-3-ol (8) is a known compound and was obtained by Corey-Bakshi-Shibata reduction of 1-hexyn-3-one. 1,4-diiodo-1,3-butadiene (9) is also a known compound and it is readily available by dimerization of acetylene accompanied by addition of iodine in the presence of platinum (IV) catalyst and sodium iodide. The last keyfragment, THP-protected 4,6-heptadiyn-1-ol (6) is a known compound.
The first step is the Sonogashira coupling of compound 8 and 9. This step gave dienynol (10) with 63 percent yield. The second step is a palladium -catalyzed coupling reaction. The coupling of compound 6 and 10 leads to the 17-carbon frame (11) with 74 percent yield. Compound 11 already has the stereo center in place and only needs a few structural changes: the third and fourth step. The third step is the reduction of the C5 triple bond in compound 11, this was accomplished by using a compound called Red-Al. The last step is the removal of the THP protection group. When THP is removed and a hydrogen is bound to the oxygen, then (R)-(–)-cicutoxin is formed. These four steps are the full synthesis of cicutoxin and gives an overall yield of 18 percent.[7]
Reactions
Cicutoxin is known to interact with the GABAa receptor and it also has been shown to block the potassium channel in T lymphocytes. A similar effect where potassium channels in neurons are blocked could account for the toxic effect on the nervous system.[10] The interactions are explained in Mechanism of action.
Available forms
Cicutoxin is found in the water hemlock. The water hemlock belongs to the Apiaceae family and species of water hemlock within this family are divided into the Cicuta and Oenanthe genera (Apiaceae also contains many other genera, and there are may edible plants in the family). The genus Cicuta contains three toxic plants: douglas water hemlock (C. douglasii), water hemlock (C. maculate), and Mackenzie’s water hemlock (C. virosa). The genus Oenanthe contains only one toxic plant the hemlock water dropwort (O. crocata).[5]
All plants from the genus Cicuta contain cicutoxin, C. virosa also contains virol A, virol C and an isomer of cicutoxin, isocicutoxin. O. crocata contains the toxin oenanthotoxin, also an isomer of cicutoxin. All of these plants contain a similar toxin. The principal structure of these toxins is a C17 conjugated polyactylene with a terminal hydroxyl group and an allylic hydroxyl group attached at C14. These toxins can be extracted from the plants named above or they can be synthesized.
Mechanism of action
The exact mechanism of action is not known for cicutoxin, even though it is well-known to be a violent toxin. The mechanism is not known because of the chemical instability of cicutoxin,[11] but there have been studies that delivered some evidence for a mechanism of action.
Cicutoxin is a noncompetitive gamma-aminobytyric acid (GABA) antagonist in the central nervous system (CNS). GABA normally binds to the beta domain of the GABAa receptor and activates the receptor which causes a flow of chloride across the membrane. Cicutoxin binds to the same place as GABA, because of this the receptor is not activated by GABA. The pore of the receptor won’t open and chloride can’t flow across the membrane. Binding of cicutoxin to the beta domain also blocks the chloride channel. Both effects of cicutoxin on the GABAA-receptor cause a constant depolarization. This causes hyperactivity in cells, which leads to seizures.[12]
There also have been some studies that suggest that cicutoxin increases the duration of the neuronal repolarization in a dose-dependent manner. The toxin could increase the duration of the repolarization up to sixfold at 100 µmol/l. The prolonged action potentials may cause higher excitatory activity[12]
It has been demonstrated that cicutoxin also blocks potassium channels in T-lymphocytes.[13] The toxin inhibits the proliferation of the lymphocytes . This has made it a substance of interest in research for a medicine against leukemia.
Metabolism
It is unknown how the body gets rid of cicutoxin. There is evidence that it has a long half-life in the body, because of a patient who was submitted in a hospital after eating a root of a Cicuta plant. The man was in the hospital for two days and still had a fuzzy feeling in his head two days after leaving the hospital.[9] There is also the case of a sheep (discussed in Effects on animals) where the sheep fully recovered after seven days.[12] This can also be explained by the structure of cicutoxin, it consists of 17 carbons, which is hydrophobic. It also has 3 double bonds, 2 triple bonds and two hydroxyl groups, which make the toxin very reactive and not easy to excrete.
Indications
First signs of cicutoxin poisoning start 15–60 minutes after ingestion and are vomiting, convulsions, widened pupils, salivation, excess sweating and the patient may go into a coma. Other described symptoms are cyanosis, amnesia, absence of muscle reflexes, metabolic acidosis and cardiovascular changes which may cause heart problems and central nervous system problems which manifest themselves as convulsions and either an overactive or underactive heart.[10][11][13] Due to an overactive nervous system respiratory failure occurs which may cause suffocation and accounts for most of the deaths. Dehydration from water loss due to vomiting can also occur. If untreated, the kidneys can also fail, causing death. Since there currently isn't any antidote for cicutoxin, the symptoms have to be treated to prevent death and/or permanent damage.[3]
Efficacy
Efficacy is the ability of a drug to reproduce a desired effect. So, although cicutoxin is a known strong poison, a study showed that it can also be used as a natural antileukemia drug.[8] The toxin inhibits the proliferation of the lymphocytes.[13] This has made it a substance of interest in research for a medicine against leukemia. In 1986 cicutoxin was first tested for its antitumor activity. This study showed that a methanolic extract of C. maculate gave a significant cytotoxicity in the 9 KB (human nasopharyngeal carcinoma) cell structure assay. These results showed the anti-leukemic effect of cicutoxin.[8] Cicutoxin is also used as a natural drug against breast cancer, because of its antitumor activity.
Adverse effects
The adverse effects are of gastrointestinal or cardiac nature; symptoms that occur most often are nausea and vomiting, excess salivation, abdominal pain, hypertension or hypotension, heart problems such as excessive or too low heartbeat, dysrhythmias, heart failure or arrest and delirium.[3] Only supportive treatment of these effects are possible, but survival rate does increase drastically due to supportive treatment. Treatments most used are administration of activated charcoal within 30 minutes of ingestion to reduce the amount of poison entering the system, keeping open the airways to prevent suffocation, rehydration to prevent dehydration by vomiting, and administration of diazepenes or barbiturates to reduce seizures.[5]
Toxicity
There are quite some known cases of toxicity caused by consumption of a Cicuta plant. Most of these cases are accidental intake of cicutoxin because the stem looks like that of a celery plant and the roots look and smell like that of parsnip. But eating a small piece of the root can be fatal. The cause of death after absorption of cicutoxin is usually respiratory failure. The LD50 of cicutoxin for mice is 2.8 mg/kg (10.8 μmol/kg). In comparison, the LD50 of virol A is 28.0 mg/kg (108 8 μmol/kg) and LD of isocicutoxin is 38.5 mg/kg (149 μmol/kg).[14] For cattle eating cicutoxin-containing plants is lethal faster than for humans, because cattle cannot vomit to reduce the amount of cicutoxin that will be absorbed.
Effects on animals
Cattle usually ingest parts of cicuta plant while they’re grazing on new growth in the spring around ditches and rivers where cicuta plants grow. Animals mostly have the same symptoms of cicutoxin poisoning as humans do, but animals do not vomit and measuring amnesia will be very hard. Symptoms that are described for animals are salivation, seizures, frequent urination and defecation and skeletal and cardiac myodegeneration. The seizures are usually short, less than a minute per seizure, with 15–30 minutes between each seizure. These seizures are spread over 2 hours after which seizures stop. The same article describes how the ewes slowly recover of its seizures and completely recovered seven days after eating the cicutoxin containing tubers.[12]
The skeletal and cardiac myodegeneration (damage of muscle tissues) only occur when a ewe was given enough cicutoxin containing tubers so as not to kill it, but will cause the intoxication symptoms. A blood inspection of the ewe showed elevated serum enzymes that indicate muscle damage (LDH, AST and CK values). At necropsy of this ewe it was found that the heart had multifocal pale areas and pallor of the long digital extensor muscle groups. The ewe that was given a lethal dose of tubers, only had microscopic lesions. The number and duration of seizures had a direct effect on the skeletal and cardiac myodegeneration and amount of serum change.[12]
The researchers also gave some ewes 1.5–2.5 times the lethal dose but gave medicines against the symptoms that occurred. They gave sodium pentobarbital (at 20–77 mg/kg intravenously) at the first seizure to control seizure activity, atropine (75–150 mg/ewe) to reduce salivary excretion during anesthesia and lactate Ringer’s solution until the ewes recovered.[12] This shows that when the symptoms are treated, it can be life-saving.
References
- ↑ Schep, L. J.; Slaughter, R. J.; Becket, G.; Beasley, D. M. G. (2009). "Poisoning due to Water Hemlock". Clinical Toxicology. 47 (4): 270–278. doi:10.1080/15563650902904332. PMID 19514873.
- ↑ M. Wink and B.-E. van Wyk (2008), Mind-Altering and Poisonous Plants of the World, p.87, Portland: Timber Press.
- 1 2 3 E.F.L.J. Anet, B. Lythgoe, M.H. Silk, S. Trippett, Oenanthotoxin and cicutoxin, Isolation and Structures, J. Chem.Soc., 1953, 309–322 doi:10.1039/JR9530000309
- 1 2 A.N.P. van Heijst, S.A. Pikaar, R.G. van Kesteren en J.M.C. Douze. Een vergiftiging door de waterscheerling (cicuta virosa). Ned Tijdschr Geneeskd 1983, 127, nr. 53
- 1 2 3 L.J. Schep, R.J. Slaughter, G. Becket, D Micheal, G. Beasley. Poisoning due to water hemlock. Clinical Toxicology (2009)
- ↑ Hazardous Substance Data Bank available at: http://toxnet.nlm.nih.gov/cgi-bin/sis/search/r?dbs+hsdb:@term+@rn+@rel+505-75-9 (accessed: 20-3-2013)
- 1 2 3 B.W. Gung, A.O. Omollo. A Concise Synthesis of R-(–)-Cicutoxin, a Natural 17-Carbon Polyenyne. European Journal of Organic Chemistry Vol. 2009, Issue 8
- 1 2 3 Takao Konoshima and Kuo-Hsiung Lee . Antitumor agents, 85. Cicutoxin, an antileukemic principle from cicuta maculata, and the cytotoxicity of the related derivatives. Journal of natural products vol 49, No 6 pp. 1117–1121 Nov–Dec 1986
- 1 2 D. Landers, K. Seppi, W. Blauer: Seizures and death on a white river float trip-Report of water hemlock poisoning. West. J. Med. 1985 May; 142:637–640
- 1 2 C.D. Klaassen (ed). Casarett and Doull's Toxicology. The Basic Science of Poisons. 6th ed. New York, NY: McGraw-Hill, 2001, p. 971
- 1 2 Uwai Koji; Ohashi Katsuyo; Takaya Yoshiaki; et al. (2000). "Exploring the Structural Basis of Neurotoxicity in C17-Polyacetylenes Isolated from Water Hemlock". J. Med. Chem. 43: 4508–4515. doi:10.1021/jm000185k. PMID 11087575.
- 1 2 3 4 5 6 K.E. Panter, D.C. Baker, P.O. Kechele. Water hemlock (cicuta douglasii) toxiceses in sheep: pathologic description and prevention of lesions and death. Journal of veterinary diagnostic intervention. Vol 8, pp 474–480, October 1996
- 1 2 3 U. Strauss, U. Wittstock, R. Shubert, E. Teuscher, S. Jung, E. Mix. Cicutoxin from cicuta virosa – a new and potent potassium channel blocker in T-lymphocytes. Miochem Biophys Res Commun. 1996 Feb. 15; 219(2):332–36
- ↑ Tomihlsa Ohta. Absolute stereochemistry of cicutoxin and related toxic polyacetylenic alcohols from cicuta virosa (1999). Faculty of Pharmaceutical Sciences, Kanazawa University, Takarmnachi, Kanazawa 920-0934, Japan
Additional References
- Hazardous Substances Data Base
- E. Anet; B. Lythgoe; M.H. Silk; S. Trippett (1953). "Oenanthotoxin and cicutoxin. Isolation and structures". J. Chem. Soc.: 309–322. doi:10.1039/JR9530000309.
- E. Anet; B. Lythgoe; M.H. Silk; S. Trippett (1952). "The Chemistry of Oenanthotoxin and Cicutoxin". Chemistry & Industry. 31: 757–758.
- K. Uwai; K. Ohashi; Y. Takaya; T. Ohta; T. Tadano; K. Kisara; K. Shibusawa; R. Sakakibara; Y. Oshima (2000). "Exploring the Structural Basis of Neurotoxicity in C17-Polyacetylenes Isolated from Water Hemlock". Journal of Medicinal Chemistry. 43 (23): 4508–4515. doi:10.1021/jm000185k. PMID 11087575.
- O.H. Knutsen; P. Paszkowski (1984). "New aspects in the treatment of water hemlock poisoning". In: Clin. Toxicol. 22 (2): 157–166. doi:10.3109/15563658408992551. PMID 6502788.