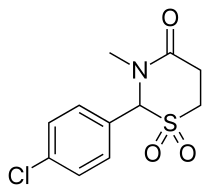
Chlormezanone
"Trancopal" redirects here. For Trancopal Dolo, see Flupirtine.
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | M03BB02 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Biological half-life | 40.5 hours |
| Identifiers | |
| |
| CAS Number |
80-77-3 |
| PubChem (CID) | 2717 |
| IUPHAR/BPS | 7323 |
| DrugBank |
DB01178 |
| ChemSpider |
2616 |
| UNII |
GP568V9G19 |
| KEGG |
D00268 |
| ChEBI |
CHEBI:3619 |
| ChEMBL |
CHEMBL1200714 |
| ECHA InfoCard | 100.001.190 |
| Chemical and physical data | |
| Formula | C11H12ClNO3S |
| Molar mass | 273.737 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Chlormezanone (marketed under the brandname Trancopal or Fenaprim) is a drug used as an anxiolytic and a muscle relaxant.
Its use was discontinued in many countries from 1996 on, due to rare but serious cases of toxic epidermal necrolysis.
Synthesis

Chlormezanone synthesis
- Sterling Drug Inc., US 3082209 (1958).
- Surrey, A. R.; Webb, W. G.; Gesler, R. M. (1958). "Central Nervous System Depressants. The Preparation of Some 2-Aryl-4-metathiazanones". Journal of the American Chemical Society. 80 (13): 3469. doi:10.1021/ja01546a065.
References
- Wollina U, Hipler U, Seeling A, Oelschlager H (2005). "Investigations of interactions of chlormezanone racemate and its enantiomers on human keratinocytes and human leucoytes in vitro". Skin Pharmacol Physiol. 18 (3): 132–138. doi:10.1159/000084910. PMID 15897685.
- Seeling A, Oelschläger H, Rothley D (2000). "Important pharmaceutical-chemical characteristics of the central muscle relaxant chlormezanone". Pharmazie. 55 (4): 293–6. PMID 10798243.
- Oelschläger H, Klinger W, Rothley D, Seeling A, Bockhard H, Hofmann B, Machts H, Riederer H, Rackur H (1998). "[Cleavage and biotransformation of the central muscle relaxant chlormezanone]". Pharmazie. 53 (9): 620–4. PMID 9770210.
- Gautier V, Vincon G, Demotes-Mainard F, Albin H (1990). "[Pharmacokinetics of chlormezanone in healthy volunteers] (original in French)". Pharmazie. 45 (4): 315–9. PMID 2399514.
This article is issued from Wikipedia - version of the 5/28/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.